Chemistry comes alive Study guide
An organic compound is analyzed, and it has twice as many hydrogen atoms as oxygen atoms. This compound is most likely
molecule with the formula C5H10O5 is a(n)
Carbohydrates have CHO with a 1:2:1 ratio
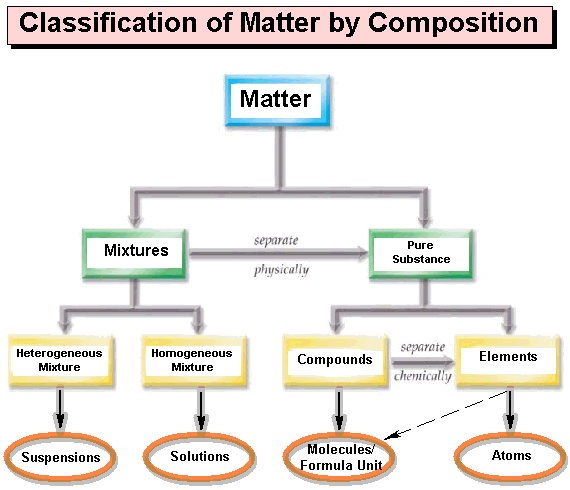
CARBOHYDRATE
- bonds are characterized by the sharing of electrons between the participating atoms.

- COVALENT
are not involved directly in the linkage between amino acids.
Removal of a water molecule between each two units
Carbohydrates and proteins are built up from their basic building blocks by the
R groups of amino acids
Organic molecules contain particular elements. Based on what you know of organic chemistry, find the mismatched pair.
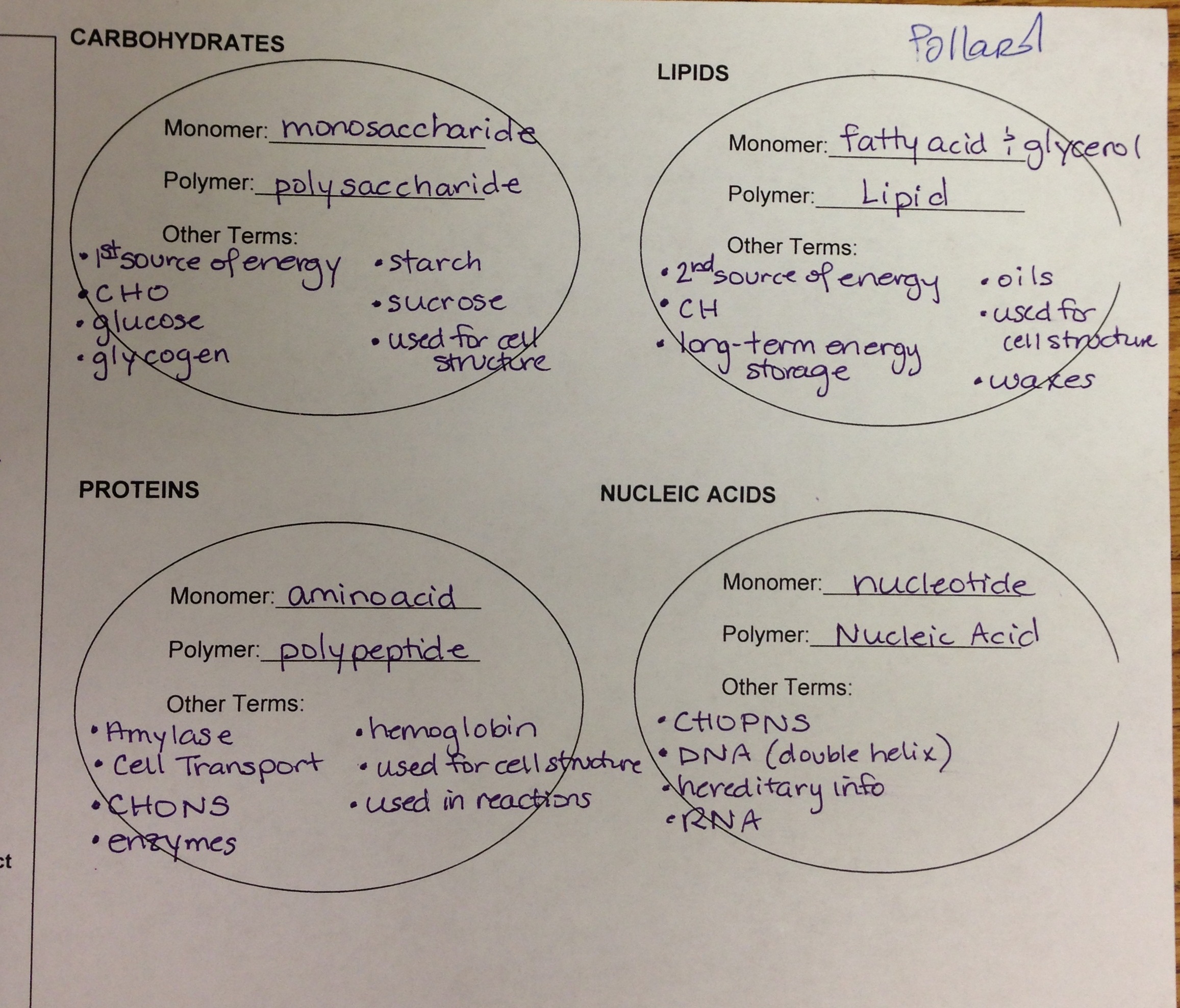
a. carbohydrate - CHO
b. lipid - CHNOP
c. protein - CHNOPS
d. nucleic acid - CHNOP
that dominate the structure of triglycerides are the basis for the efficient and compact energy storage by fat.
hydrocarbon groups
The single most abundant protein in the body is
collagen
An electron is a(n)
negatively charged subatomic particle
The function of microRNA (miRNA) is to
T urn some genes on and others off, thus controlling genetic expression
Simple sugars are also known as...
monosaccharides
which ranges from 0 to 14, is a logarithmic scale based on the concentration of hydrogen ions in a solution.
pH scale

Organic chemistry is the chemistry of a.

carbon compounds
The sequence of amino acids in a protein constitutes the __________ structure of the protein.
Primary
Atomic number is determined by the number of ___ in each atom.
protons only
DNA and RNA are nucleic acids
they are built from nucleotides
An atom or a group of atoms possessing a positive or negative electrical charge is called?
ION
Prostaglandins are
type of eicosanoids, which are diverse lipids found in the cell membrane.
are linked by peptide bonds to form polypeptides.
Amino acids

- 14Carbon has an atomic number of 6, but has 8 neutrons. How many electrons are present in each atom?

- 6
The space between the pleurae of the lungs that
extends from the sternum to the
vertebral column is
the
mediastinum
A pentose sugar, a phosphate group and a nitrogen-containing base are found in a(n)
NUCLEOTIDE
All isotopes of a particular element possess

the same number of protons, but different numbers of neutrons

Which of the following is a condensation (dehydration synthesis) reaction?

the breakdown of a polymer into monomers
Which of the following types of energy moves in waves?
Radiant energy moves in waves
Aerobic cellular respiration occurs in the
RIBOSOMES

Which protein types are vitally important to cell function in all types of stressful circumstances?
molecular chaperones
- Electrically charged particle due to loss of an electron.
- Neutral subatomic particle.
- Smallest particle of an element that retains its properties.
- Smallest particle of a compound that still retains its properties
- Cation
- Neutron
- Atom
- Molecule
Detoxification of certain potentially poisonous molecules occurs in the
peroxisomes

This organelle is the command and control center of the cell
nucleus
- Molecular chaperones aid in the desired folding of
- are formed from amino acid building blocks
- Amino acids are the building blocks or monomers for
- Which organic molecules form the major structural materials of the body?
- all enzymes that have been identified are
- The basic structural material of the body consists of
- PROTEIN
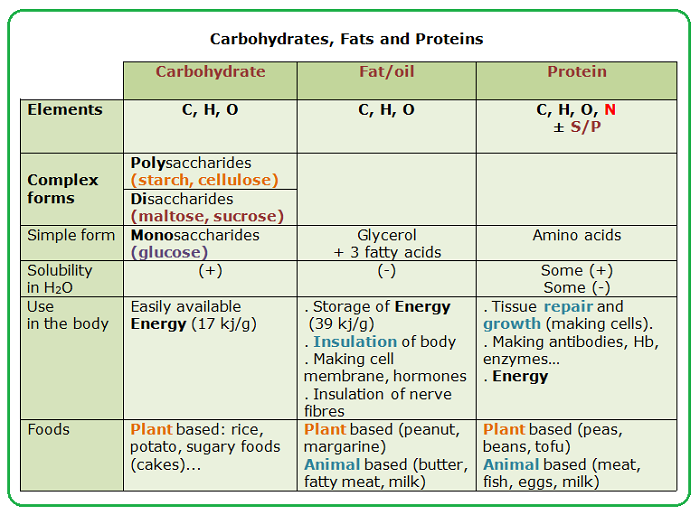
- Which of the following would be regarded as an organic molecule?

- CH4
The difference between a colloid and a suspension is
colloid can undergo sol-gel transformation, whereas a suspension cannot.
- The mitochondria are organelles that
- Which of the following is the major positive ion outside cells?
- produce ATP from the chemical energy of food
- Sodiun (NA)
Identify the mismatched pair
a. ribosome synthesis – nucleolus
b. rough endoplasmic reticulum – attached ribosomes
c. Golgi apparatus – lytic enzymes
d. cytoskeleton – microtubules

Proteins that are secreted outside the cell in the process of exocytosis are released by

secretory vesicles
Cilia and flagella are specialized structures used
for locomotion
- Atom X has 17 protons. How many electrons are in its valence shell?
- The linkage between amino acids is a type of .
- 7
- covalent bond
Why are ribosomes attached to the endoplasmic reticulum (ER)?
Newly synthesized polypeptides can move directly through the ER membrane.
Which of the following is an example of a suspension?
BLOOD
Enzymes are important as
catalysts for chemical reactions
What is activation energy?
the energy required to start a chemical reaction
Enzymes?
lower activation energy requirement
The reactants in an enzyme catalyzed reaction are called?
substrates

The information about the synthesis of enzymes comes from
DNA (Deoxyribonucleic acid)
Lungs, heart, or brain are examples of
organs
Circulatory, respiratory, or digestive are examples of
systems

Diffusion is the process by which a substances moves from an area of
higher concentration to an area of lower concentration
A diffusion equilibrium is reached when
there is no net movement between two areas
Osmosis is a special case of
diffusion that involves the movement of water across a membrane
The net movement of water across a selectively permeable membrane is always
from the hypotonic solution to the hypertonic one

The direction of osmosis is determined by the
concentration of dissolved solutes on both sides of the membrane.
is the process by which the undigested contents of food vacuoles are removed from the cell.
Exocytosis
A cell placed in a beaker of sea water will
shrink due to the loss of water by osmosis
When a neuron is stimulated and Na+ ions move through an open Na+ channel, the process is
facilitated diffusion
A hypothetical “microbullet” shot through a phospholipid bilayer would pass the components in which order?
a. polar >>> nonpolar>>>polar>>>nonpolar
b. polar>>>polar>>>nonpolar>>>nonpolar
c. nonpolar>>>polar>>>polar>>>nonpolar
d. polar>>>nonpolar>>>nonpolar>>>polar
Identify the mismatched pair:
a. hydrophilic – polar molecules
b. hydrogen ion – proton
c. acid solution – pH more than 7
d. water – universal solvent
Which of the following statements is true about a solution with a pH of 9, one with a pH of 2 and one with a pH of 7?
The pH 2 solution has a higher hydrogen (H+) ion concentration than the pH 7 solution.

The relationship between mass and volume could best be described as

Increasing volume results in an increase of mass
An atom whose atomic number is 10 has how many electrons in its outermost energy level?
8
Isotopes are atoms of the same element that differ in their
Number of Neutrons

Which of the following are found in the nucleus of an atom?

Protons & Neutrons

An element with 22 protons, 22 neutrons, and 22 electrons would have an atomic number of

22

Which of the following results from the making of a bond?
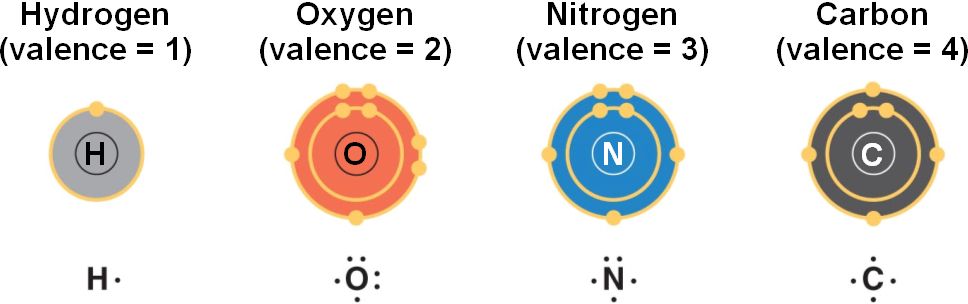
Atoms become more stable
For an atom to be considered an ion
Protons can outnumber electrons

An atom becomes an ion when

It gains or loses electrons
Hydrogen bonds are very important in the functional shape of
Proteins & Nucleic acids

The symbol 3CO2 represents

3 molecules of carbon dioxide

In a bottle of water, hydrogen bonding occurs between the hydrogen of 1 atom and
An oxygen atom in a different molecule
Which of the following is not a compound?
Sodium
Atoms form bonds to
Fill their outer shells with electrons
Water is a polar molecule because
Oxygen is more electronegative than hydrogen
Potassium has one electron in its fourth shell, and chloride has seven electrons in its third shell. Which of the following is most likely to be true?
Potassium will give an electron to chloride to form an ionic bond
You mix sugar in water and stir until it’s completely dissolved. In this system, the water is the ______, the sugar is the______ and the end result is a _______.
Solvent; solute; solution

Each element has a unique_________, which refers to the number of protons present in its atoms.

Atomic number
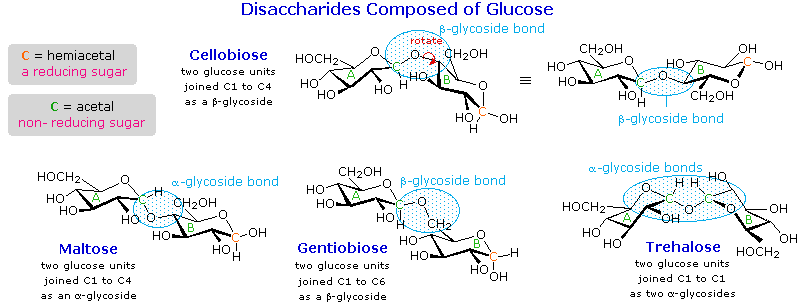
A molecule is ________.

A bonding together of two or more atoms
If lithium has an atomic number of 3, and an atomic mass of 7, it has________ neutron(s) in its nucleus.

4
Substances that are nonpolar and repelled by water are ________.

Hydrophobic
A hydrogen bond is _________.
Formed when an electronegative atom of a molecule weakly interacts with a hydrogen atom that is already participating in a polar covalent bond

An ionic bond is one in which ________.

Two charged atoms have a mutual attraction due to electron transfer or donation
A covalent is one in which?
Two charged atoms have a mutual attraction due to electron transfer
Lipids ______.
Include triglycerides that serve as energy sources
is energy directly involved in moving matter.
Mechanical energy
DNA _______.
Contains protein-building instructions
The building blocks of RNA and DNA are
nucleotides

A nonpolar covalent bond implies that ________.

There is no difference in charge at the ends (the two poles) of the bond
A solution with a pH of 11 is ______ times as basic as one with.
1,000
Carbon is part of so many different substances because _______
A carbon atom generally forms four covalent bonds with a variety of atoms
A solution with a pH of 4 has_________.
More H+ ions than OH- ions
Hydrolysis could be correctly described as the ________
Breaking of a long-chain compound into its subunits by adding water molecules to its structure between the subunits Process of condensation in reverse.

Genetic instructions are encoded in the base sequence of________; molecules of _________ function in processes using genetic instructions to construct proteins

DNA;RNA
Membranes consist of ___________.
a. A phospholipid bilayer
b. proteins c. glycolipids and glycoproteins
d. cholesterol
e. all of the above are correct
The nucleolus is the site where
The protein and RNA subunits of ribosomes are assembled
Which of the following forms of energy travels in varying wavelengths, such as visible light, infrared waves, radio waves, ultraviolet waves, and X-rays?
electromagnetic energy
- refers to radiant light and heat from the sun.
- Solar energy
The _________ is free of ribosomes and curves through the cytoplasm like connecting pipes; the main site of lipid synthesis.
Smooth ER
Mitochondria convert energy stored in ______ to forms that the cell can use, principally ATP.
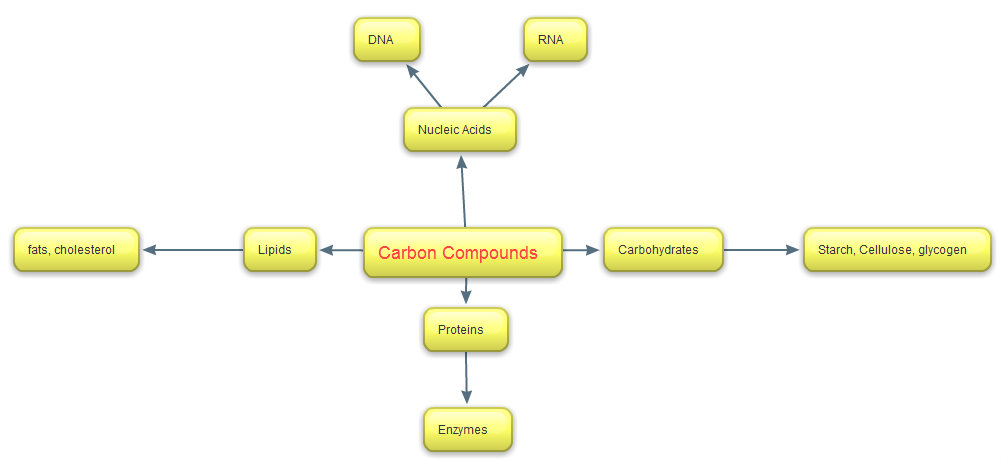
Carbon compounds
______are sacs of enzymes. They digest or break down worn out organelles so that the material can be recycled and reused by the cell.
Lysosomes
Two classes of cytoskeletal elements underlie nearly all movements of eukaryotic cells; they are __________
Microtubules and microfilaments

White blood cells use________ to devour disease agents invading your body.

Phagocytosis

In a lipid bilayer, tails point inward and form a(n) _______ region that excludes water.
Hydrophobic
Which of the following is not a form of active transport?
Bulk flow
Which of the following is not a form of passive transport?
Exocytosis
O2, CO2, H2O, and other small, electrically neutral molecules move across the cell membrane by________.
Passive transport
Ions such as H+, Na+, K+, and Ca++ move across cell membranes against its concentration gradient by_________.
Active transport
An enzyme is best described as _________.
Protein & Fat
Which is not true of enzyme behavior?
An individual enzyme can catalyze a wide variety of different reactions
- When NAD+ combines with hydrogen, the NAD+ is __________.
- A substance that gains electrons is ________.
- Reduced
As to major function, NAD+, FAD, and NADP+ are classified as _________.
cofactors that function as coenzymes
When a phosphate bond is linked to ADP it then makes ATP, this bond __________.
releases a large amount of usable energy when the phosphate group is split off during hydrolysis
Glycolysis would quickly halt if the process ran out of _________, which serves as the hydrogen and electron acceptor.
NAD+ Nicotinamide adenine dinucleotide
The ultimate electron acceptor in aerobic respiration is _________.
Oxygen (O2)
When glucose is used as an energy source, the largest amount of ATP is generated by the ________ portion of the entire respiratory process.
electron transport chain

What is the name of the process by which reduced NADH transfers electrons along a chain of acceptors to oxygen so as to form water and in which the energy released along the way is used to generate ATP?

the electron transport chain

Pyruvic acid can be regarded as the end product of _________.

glycolysis
ATP (Adenosine triphosphate) production by chemiosmosis involves __________.
a. H+ concentration and electric gradients across a membrane
b. ATP (Adenosine triphosphate) synthesis
c. formation of ATP in the inner mitochondrial compartment
d. all of the above
During the fermentation pathways, a net yield of two ATP is produced from __________; the NAD+ necessary for _________ is regenerated during the fermentation reactions.
glycolysis; glycolysis
The replication of DNA occurs
between the growth phase of interphase

is o carry out the genetic instruction (provided by DNA) for protein synthesis
major function of RNA
Diploid refers to ______.
having two chromosomes of each type in somatic cells
Somatic cells are __________ cells; germ cells are _________ cells.
BODY; MEIOTIC
If a parent cell has sixteen chromosomes and undergoes mitosis, the resulting cells will have__________ chromosomes.
c. sixteen
The correct order of the stages of mitosis is.
prophase, metaphase, anaphase, telophase
The nuclear envelope breaks completely into numerous tine, flattened vesicles. Now the chromosomes are free to interact with microtubules that are extending toward them, from the poles of the forming spindle.” These sentences describe the __________ of mitosis.
prophase
During ______, sisters chromatids of each chromosome are separated from each other, and those former partners, now chromosomes move to opposite poles.
anaphase
In the process of cytokinesis, cleavage furrows are associated with _______ cell division, and cell plate formation is associated with ______ cell division.
animal; plant
Each DNA strand has a backbone that consists of alternating ________.
sugar and phosphate molecules
In DNA, complementary base-pairing occurs between.
adenine and thymine

The chemical symbol O:O means.
the atoms are double bonded
Adenine and guanine are _________.
double-ringed pyrimidines
- in which electrons are shared unequally is termed a polar covalent bond.
- covalent bond

Transcription _________.

occurs during the synthesis of any type of RNA by use of a DNA template
________ carry(ies) amino acids to ribosomes, where amino acids are linked into the primary structure of a polypeptide.
tRNA Transfer ribonucleic acid

- The RNA responsible for bringing the amino acids to the "factory" site for protein formation is the
- tRNA
Transfer RNA differs from other types of RNA because it ___.
carries an amino acid at one end
- The important cholesterol-based molecules are

- steroids

______ and ______ are found in RNA but not in DNA.
Uracil; ribose

You notice that you cannot read your book through a test tube of patient fluid held against the print, making it so blurred as to be unreadable. There is no precipitant in the bottom of the beaker, though it has been sitting for several days in a rack. What type of liquid is this?

colloid
are built from nucleotide building blocks.
Nucleic acids
are two of the three major types of lipids.
Triglycerides and phospholipids
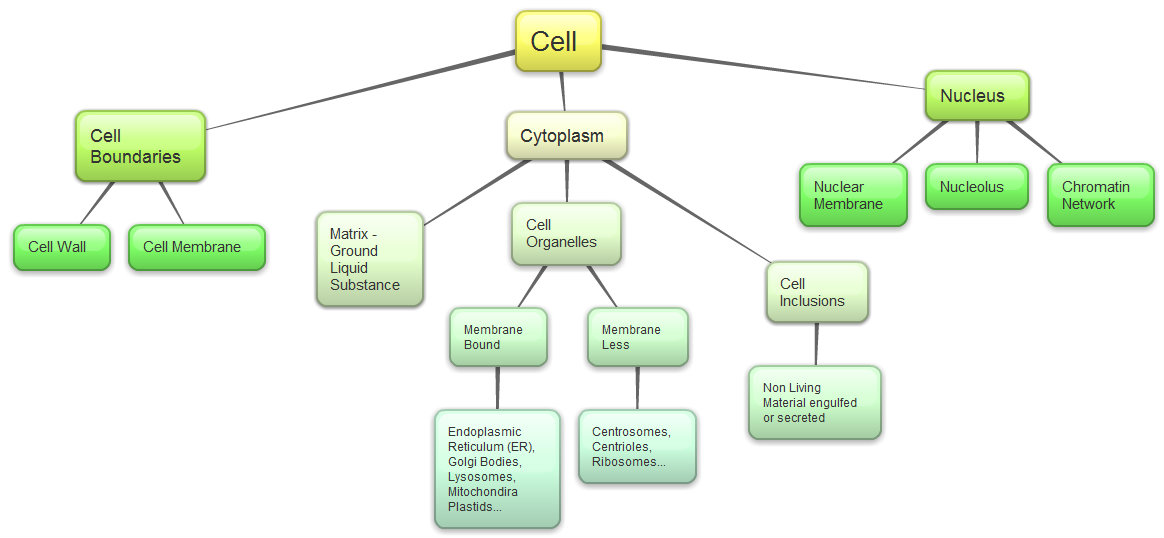
- are built from monosaccharide building blocks.

- Carbohydrates

- The genetic information is coded in DNA by the.
- is atomic number 6 and has four electrons in its outermost (valence) electron shell.

- sequence of the nucleotides
- Carbon

Which of the following is not true of proteins?

Their function depends on their three-dimensional shape.
They appear to be the molecular carriers of coded hereditary information.
They have both functional and structural roles in the body..
They may be denatured or coagulated by heat or acidity.

- TRUE regarding the pH of a solution?

- Proteins
- The more hydrogen ions in a solution, the more acidic the solution is.
contains a carboxyl group (-COOH), which may ionize to form an acidic solution.
R group of amino acid
- Foods are broken down into their building blocks by adding water. This would be an example of which characteristic of water?
- Buffers tend to prevent dramatic changes in the pH when __________ are added to a solution.
- Water acts as a reactant.
- proton donors or proton acceptors
- If atom X has an atomic number of 74 it would have which of the following?
- Select the correct statement about isotopes.
- The chemical symbol O=O means
- 74 protons
- Isotopes of the same element have the same atomic number but differ in their atomic masses.
- the atoms are double bonded
- Which of the following is a property of matter?
- is determined by the total number of protons and neutrons
- Matter can exist in solid, liquid, or gaseous states.
- Atomic mass
Which of the following is the primary energy-transferring molecule in cells?
ATP
are necessary for salivary amylase to hydrolyze starch to glucose in your mouth. Thus, water acts as an important reactant.
water molecules
- Which of the following is NOT considered a form of matter?
- X rays are a type of energy.
Carbohydrates and proteins are built up from their basic building blocks by the
removal of a water molecule between each two units
What level of protein synthesis is represented by the coiling of the protein chain backbone into an alpha helix
secondary structure
In plasma, a typical body fluid, protein floating around would be considered to be which of the following?
a solute, specifically both a colloid and an electrolyte.Colloids are large particles dispersed in body fluids. Since most proteins have a negative charge, they are also considered to be electrolytes.
Cortisol is a type of lipid hormone. Which type of lipid would cortisol be classified as?
cortisol is a steroid hormone synthesized from cholesterol.
Electrolytes are charged particles called ions that are dissolved in body fluids. Which of the following ions would be considered a major anion in the body?
chloride is a major extracellular anion (negative ion).
Cell junctions that promote the coordinated activity of cells by physically binding them together into a cell community include all of the following except?
peroxisomes
Which statement about enzymes is false?
Enzymes raise the activation energy needed to start a reaction.
which are capable of conducting an electrical current?
ions, which are capable of conducting an electrical current.
is a reactant in an enzyme-catalyzed reaction.
substrate
Which of the following does NOT represent an accurate hierarchy in the natural combination of matter?
When two or more similar kinds of atoms bind, they form an isotope.
In order for the DNA molecule to get "short and fat" to become a chromosome, it must first wrap around small molecules called
histones
- Sugars contain all & EXCEPT
.
- CHO- carbon hydrogen oxygen EXCEPT calcium
- Some hormones enter cells via
- Sucrose is a
- receptor-mediated endocytosis
- disaccharide
Which of the following is true regarding the generation of a membrane potential?
Both potassium and sodium ions can "leak" through the cell membrane due to diffusion
Transcytosis is?
transporting an endosome from one side of a cell to the other and releasing the contents by exocytosis
- The drug Procaine (also known as Novocaine) blocks sodium (Na+) channels in plasma membranes. Given this information, which one of the following statements is most likely true about a cell bathed in a solution that contains Procaine?
- Na+ will be unable to cross the membrane by facilitated diffusion.
- Why are free radicals so dangerous to cells, and how are they dealt with by the body?
- Free radicals are highly reactive chemicals that cause havoc in any cellular environment by reacting with things they should not. Cells with peroxisomes have enzymes specific to reducing free radicals into less reactive chemicals.
- Which of the following is not an electrolyte?
- HCO3-.
- H2O
- a bicarbonate ion