Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Exam 1 questions
front 1 How many moles of NaOH are required to titrate 0.1 mol of acetic acid (pKa = 4.76) to its equivalence point? | back 1 0.1 mol of NaOH |
front 2 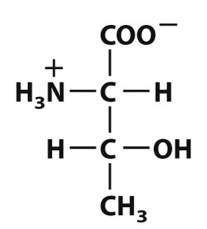 Which of the following defines the polar functional groups present in this amino-acid structure? | back 2 Amino group, carboxyl group, and hydroxyl group |
front 3 Which of the following is a pyrimidine nucleotide component of RNA? | back 3 Uridine monophosphate |
front 4 Two reactions are coupled, both in the forward direction. One has ΔG°' = -25 kJ mol-1 and the other has ΔG°' = 15 kJ mol-1. What is the net ΔG°' for the coupled process? | back 4 -10 kJ mol-1 |
front 5 Which statement best describes how water can participate in a buffer system? | back 5 Water can support both a weak acid and its conjugate base, due to its ability to dissociate into H⁺ and OH⁻. |
front 6 All of the following statements regarding DNA sequencing are correct, EXCEPT: A) DNA sequencing by dideoxy chain termination exploits the chemistry of DNA replication in living cells. B)The products with an incorporated ddNTP are detected by fluorescence. C) DNA polymerase will not catalyze nucleotide addition in the absence of an annealed primer. D) The sequence of a DNA sample is deduced from the different fluorescent colors of the 5' phosphate on each primer strand. E) Chain termination operates as a random process: not every polymerization step incorporates a ddNTP. | back 6 D) The sequence of a DNA sample is deduced from the different fluorescent colors of the 5' phosphate on each primer strand. |
front 7 What causes the hydrophobic effect in aqueous solutions? | back 7 Water molecules forced to order in a structured "cage" around nonpolar molecules. |
front 8 Why do ice and liquid water have different densities? | back 8 The arrangement of water molecules in ice forms an open lattice structure, making ice less dense. |
front 9 Which element is central to organic molecules due to its ability to form four covalent bonds? | back 9 Carbon |
front 10 The second law of thermodynamics states: | back 10 The entropy of the universe is constantly increasing. |
front 11 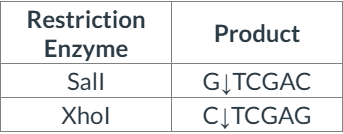 Consider the pair of restriction enzymes below and their products of restriction digestion. Which of the following statements is correct? | back 11 The enzymes produce identical sticky ends, with 5' overhangs. |
front 12 What is the equilibrium constant (Keq) for a reaction with the following parameters? ΔG°' = -5.7 kJ mol-1 T = 25°C | back 12 10 |
front 13 For a biochemical reaction at 37°C, if the equilibrium constant (Keq) is 50, what is the ΔG°' for the reaction? | back 13 -10.1 kJ mol-1 |
front 14 All of the following are physical properties of water, EXCEPT: A) Excellent solvent properties B) High heat capacity C) Less dense as a liquid D) High boiling point E) Cohesion and adhesion | back 14 C) Less dense as a liquid |
front 15 What is the ΔG (Gibbs free energy) for a reaction with the following parameters? ΔH = -40 kJ mol-1 ΔS = -0.1 kJ mol-1 K-1 T = 298 K | back 15 -10 kJ mol-1 |
front 16 What type of bond is formed between the phosphate group of one nucleotide and the hydroxyl group of the sugar in another nucleotide? | back 16 Phosphoester bond |
front 17 Which nucleotide is the primary source of energy transfer reactions in cells? | back 17 ATP |
front 18 Which of the following statements describes DNA synthesis? | back 18 The pyrophosphate product is hydrolyzed for energy, to drive the reaction forward. |
front 19 Which type of bond is most commonly involved in maintaining the three-dimensional structure of nucleic acids? | back 19 Hydrogen bonds |
front 20 Which of the following describes an amide bond? | back 20 Formed between a carboxyl group and an amino group |
front 21 Which of the following best describes an endergonic reaction? A) It releases free energy B) It occurs spontaneously C) It has a ΔG < 0 D) It involves the transfer of electrons E) It requires an input of energy to proceed | back 21 It requires an input of energy to proceed |
front 22 A newly sequenced genome consists of 31% thymine. Using Chargaff's rules for base pairing, what must be the GC content for this genome? | back 22 38% |
front 23 Which functional group is present in alcohols and is responsible for their hydrophilic properties? | back 23 Hydroxyl group (-OH) |
front 24 Given the following parameters, what is the pH of the buffer solution? 0.2 M acetic acid 0.2 M acetate. pKa of acetic acid is 4.76 | back 24 4.76 |
front 25 Which of the following scientists below recognized the helical structure of DNA based on X-ray diffraction images? | back 25 Francis Crick |
front 26 Which buffer would be most effective at maintaining a pH of 7.4 if you have access to acetic acid (pKa = 4.76) and phosphoric acid (pKa = 7.2)? | back 26 Phosphoric acid |
front 27 What differentiates a nucleoside from a nucleotide? | back 27 The addition of one or more phosphate groups |
front 28 Which of the following statements describes the structure of DNA? A) Nature chose to replace the 2' OH with H for stability, but it could have been the 3' hydroxyl replaced with H, instead. B) A 2' hydride and a methylated version of adenine's base-pair partner allow for greater stability of this storage form of nucleic acids. C) The nucleotides from one strand are linked to the nucleotides of the other strand through phosphodiester bonds. D) The hydrogen bonds in GC are shorter than in AT. E) The enol tautomer and anti-conformation predominate in the double helix at pH 7. | back 28 B) A 2' hydride and a methylated version of adenine's base-pair partner allow for greater stability of this storage form of nucleic acids. |
front 29 Which of the following makes “palindromic,” double-stranded DNA? A) GATATC B) TAGGTA C) CATGTG D) TTGGAA E) GACCTC | back 29 A) GATATC |
front 30 Which functional group serves as a hydrogen bond donor from adenine, when it is part of a base pair? | back 30 Amino (-NH2) |
front 31 Which nucleotide contains a ribose sugar and adenine base? | back 31 ATP |
front 32 Which of the following statements about entropy is true? A) ΔS = 0 at 0 °C B) Biological polymers have more entropy than their inorganic precursors. C) Ice has a more positive entropy than liquid water. D) A reaction with a negative entropy is endergonic. E) Dialysis is driven by entropy. | back 32 Dialysis is driven by entropy. |
front 33 All of the following are common nitrogenous base found in nucleotides, EXCEPT: A) Cytosine B) Adenine C) Thymine D) Uracil E) Guanosine | back 33 Guanosine |
front 34 Water molecules participate in hydrogen bonding with the following functional groups, EXCEPT: A) amino groups B) methylene groups C) carboxyl groups D) hydroxyl groups E) All of these form hydrogen bonds with water | back 34 B) methylene groups |
front 35 The standard free energy change (ΔG°') for the hydrolysis of ATP to ADP and Pi is -30.5 kJ/mol. If 1 mole of ATP is hydrolyzed, what is the overall change in free energy? | back 35 -30.5 kJ |
front 36 Using the convention for writing nucleic acid sequences, what is the sequence of DNA complementary to the sequence, below? AGATCGGTATCAGGT | back 36 ACCTGATACCGATCT |
front 37 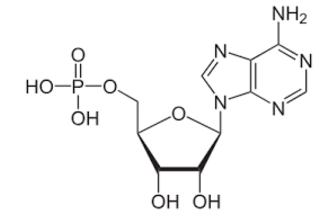 What is the correct name for the structure below? | back 37 Adenosine monophosphate |
front 38 Thymine contains which functional group that participates as a hydrogen bond acceptor in DNA? | back 38 Carbonyl (C=O) |
front 39 Given the parameters for the reaction below, solve for Q. 2E + F --> G T = 37oC ΔG°’ = 4.3 kJ mol-1 ΔG' = 23 kJ mol-1 | back 39 1.4 x 103 |
front 40 Which of the following is true regarding the ionization of water? | back 40 Water has a very low degree of ionization, forming equal concentrations of H⁺ and OH⁻ ions at 10⁻⁷ M each. |
front 41 All of the following are considered one of the four major classes of biomolecules, EXCEPT: A) lipids B) carbohydrates C) nucleic acids D) amino acids E) proteins | back 41 D) amino acids |
front 42 After chain termination, the sequencing products are separated by gel electrophoresis based on_____, via migration from the______electrode to the______electrode. | back 42 length, negative, positive |
front 43 Given a reaction with ΔG' = 15 kJ mol-1, what can be said about the reaction? | back 43 Non-spontaneous |
front 44 In an exergonic reaction, the Gibbs free energy (ΔG) is | back 44 Negative |
front 45 A buffer contains 0.1 M lactic acid (pKa = 3.86) and 0.05 M sodium lactate. What is the pH of the buffer? | back 45 3.56 |
front 46 What is the primary function of a restriction enzyme in DNA analysis? | back 46 To cleave double-stranded DNA at specific sequences |
front 47 Identify the functional group(s), in the following chemical structures, that contribute to buffering capacity: R-COOH | back 47 Both carboxyl group (−COOH) and carboxylate group (−COO−) |
front 48 What type of ends do some restriction enzymes generate after cutting DNA? | back 48 Sticky ends |
front 49 Which property of water allows it to dissolve a wide variety of solutes? | back 49 Its polar nature and ability to form hydrogen bonds |
front 50 Due to 2, consecutive hydroxyls on the carbon backbone (vicinal diol), ______ is vulnerable to hydrolysis, compared to its counterpart in DNA, ______, which has one of the vicinal hydroxyls removed. The synthetically-designed ______ has both vicinal hydroxyls removed, resulting in chain termination, during DNA polymerization. | back 50 ribose, deoxyribose, dideoxyribose |