Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Ch 8 notecards
front 1 The smallest sugars are | back 1 aldoses or ketose |
front 2 Monosaccharides cyclize to form | back 2 alpha or beta anomers |
front 3 The derivatives of monosaccharides include | back 3 aldonic acids, uronic acids, alditols, deoxy sugars, and amino acids |
front 4 What links monosaccharides to other molecules? | back 4 glycosidic bonds |
front 5 Monosaccharides are ______ from smaller precursors that are derived from _______ by photosynthesis | back 5 synthesized, CO2 and H2O |
front 6 Carbohydrates are classified into three groups: | back 6 Monosaccharides, oligosaccharides, polysaccharides |
front 7 Monosaccharides are not broken down into | back 7 simple sugars under mild conditions |
front 8 Oligosaccharides usually have | back 8 2 to 10 simple sugar residues |
front 9 Polysaccharides are | back 9 polymers of simple sugars |
front 10 What functional groups do Aldose and ketone have in common? | back 10 An aldehyde and ketone functional group |
front 11 Examples of chiral monosaccharides: | back 11 -Aldose with 3 or more carbon atoms - Ketone with 4 or more carbon atoms |
front 12 How do you know if it has a D or L configuration? | back 12 look at the highest-numbered chiral center |
front 13 what structure does Gram-negative have? | back 13 two membranes with a thin peptidoglycan shell |
front 14 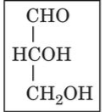 | back 14 D-glyceraldehyde |
front 15  | back 15 D-ribose |
front 16 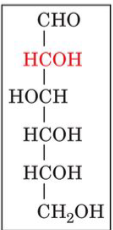 | back 16 D-glucose |
front 17 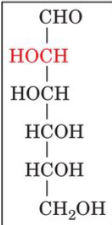 | back 17 D-mannose |
front 18 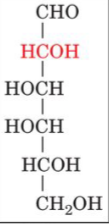 | back 18 D-galactose |
front 19 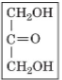 | back 19 dihydroxyacetone |
front 20 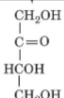 | back 20 D-Erythrulose |
front 21 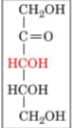 | back 21 D-ribulose |
front 22 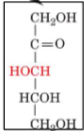 | back 22 D-Xylulose |
front 23 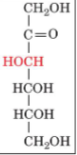 | back 23 D-fructose |
front 24 What configuration predominates in nature? | back 24 D-sugars |
front 25 Starch and glycogen are (homopolysaccharides) and their job is | back 25 storage of molecules |
front 26 Isomers that have opposite configuration at one or more chiral centers but not mirror images are | back 26 diastereomers |
front 27 Sugars that differ only by one chiral center are | back 27 epimers |
front 28 Pyranose (6-membered ring) | back 28 cyclic form of glucose |
front 29 Furanose (5-membered ring) | back 29 cyclic form of fructose |
front 30 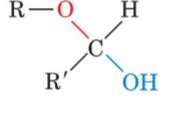 Glucose(an aldose) can cyclize for form a | back 30 cyclic hemiacetal |
front 31 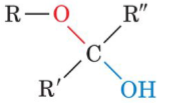 Fructose (a ketone) can cyclize to form | back 31 cyclic hemiketal |
front 32 When hemiacetals and hemiketals are formed, the carbonyl atoms becomes a | back 32 new asymmetric center |
front 33 When OH bond on carbon 1 if down it's | back 33 alpha |
front 34 When OH bond on carbon 1 if up it's | back 34 beta |
front 35 Isomers of monosaccharides that differ only in their configuration in the asymmetric carbon are called | back 35 anomers |
front 36 Sugar alcohols are formed by | back 36 mild reduction of sugars |
front 37 Sugar esters phosphate esters are important for | back 37 energy like ATP |
front 38 Disaccharides are the simplest oligosaccharides with | back 38 2 monosaccharides linked by glycosidic bond |
front 39 Each unit in an oligosaccharide is termed as a | back 39 residue |
front 40 reducing sugar can open to | back 40 linear form and undergo reduction |
front 41 Sucrose is not a reducing sugar because | back 41 it doesn't have a free anomeric carbon |
front 42 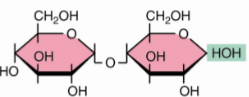 | back 42 Maltose α(1→4) |
front 43 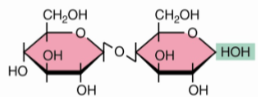 | back 43 Cellobiose β(1→4) |
front 44 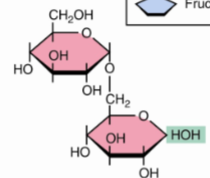 | back 44 Isomaltoseα(1→6) |
front 45 Are composed of the vitreous humor of the eye and is the lubricant fluid for joints | back 45 Hyaluronates |
front 46 A polysaccharide made up of different monosaccharides | back 46 heteropolysaccharide |
front 47 Starch and glycogen are (homopolysaccharides) and their job is | back 47 storage molecules |
front 48 Chitin and cellulose are (homopolysaccharides) and they are | back 48 structural molecules |
front 49 cell surface polysaccharides' job is | back 49 recognition of molecules (hetero) |
front 50 Starch has 2 forms | back 50 amylose and amylopectin |
front 51 Amylose has a _____links, no branches and____reducing end | back 51 α(1→4), one |
front 52 Amylopectin branches are______ every 12-30 residues, ____reducing end | back 52 α(1→6), one |
front 53 Amylose has poor solubility in water and has a | back 53 helical and hollow shape |
front 54 Iodine can fit into the hydrophobic middle of | back 54 amylose |
front 55 the more branches, the more sites for | back 55 phosphorylase to release glucose 1-P |
front 56 glycogen is the glucose (energy) storage device in | back 56 animals |
front 57 Glycogen has____ backbone, ____branches every 8-12 branches | back 57 α(1→4), α(1→6) |
front 58 When iodine is added to glycogen, | back 58 it becomes a red-violet color |
front 59 Dextrans formed by bacteria are components of | back 59 dental plaque |
front 60 Amylose prefers a____ conformation due to its bent α(1→4) linkages | back 60 helical |
front 61 Cellulose with beta(1→4) linkages can adopt an ____ conformation | back 61 extended |
front 62 Is a structural polysaccharide, most abundant polymer, and found in plant cell walls | back 62 Cellulose |
front 63 Cellulose has | back 63 Interchain and Intra chain H-bonds |
front 64 Is found in the exoskeletons of crustaceans, insects, spiders, and fungi walls | back 64 chitin |
front 65 Cellulose strand are | back 65 parallel |
front 66 Chitin has both | back 66 antiparallel and parallel strands |
front 67 Has repeating disacchairde with amino sugars and negative charge | back 67 glycosaminoglycans |
front 68 Heparin has a very high negative charge and is a natural | back 68 anticogulant |
front 69 Are found in tendons, cartilage, and connective tissues | back 69 chondroitin and keratan |
front 70 Proteoglycans are large | back 70 glycosaminoglycan-containing proteins |
front 71 Bacterial cell walls consists of glycan chains cross-linked by peptides | back 71 peptidoglycans |
front 72 The oligosaccharide chains covalently attached to eukaryotic proteins play a role in protein structure and recognition | back 72 glycoproteins |
front 73 N-linked carbohydrates are attached to side-chain amide nitrogen of | back 73 asparagine residues on glycoproteins |
front 74 O-linked carbohydrates are attached to side chain hydroxyl groups of | back 74 serine residues on glycoproteins and proteoglycans |
front 75 How do proteoglycans and glycoproteins differ? | back 75 proteoglycans have O-linked glycosaminoglycans and glycoproteins have N-linked olgiosaccharides |
front 76 Proteoglycans are components of | back 76 animal cells, membranes and glycocalyx |
front 77 Proteoglycans are soluble protein components of the | back 77 extracellular matrix outside the cell membrane |
front 78 Functions of proteoglycan | back 78 1. Modulation of cell growth 2. Cushioning in joints |
front 79 Gram-positive has | back 79 one membrane with a thick peptidoglycan outer shell |
front 80 Gram-negative has | back 80 two membranes with a thin peptidoglycan shell |
front 81 Gram-negative cells have "hairy" | back 81 lipopolysaccharide |
front 82 lipopolysaccharide consists of | back 82 lipid group joined to polysaccharide and create monosaccharide chain |
front 83 The three types of N-linked glycoproteins: | back 83 high mannose, complex, and hybrid |
front 84 Can alter chemical and physical properties of proteins, stabilize protein conformation, and cleave monosaccharide units from N-linked glycoproteins | back 84 oligosaccharides |