Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Protein Degradation iRAT
front 1 Why is protein degradation important? | back 1 It allows the cell to have a constant supply of amino acids and prevents the accumulation of abnormal proteins. |
front 2 Short-lived proteins with a half-life with seconds and minutes are degraded by what pathway? | back 2 Cytosolic ATP dependent pathway |
front 3 Cytosolic ATP dependent pathway is mediated by- | back 3 the proteosome |
front 4 What is the function of the proteosome? | back 4 carries out hydrolytic cleavage |
front 5 What degrades proteins with long half lives? | back 5 Lysosomes |
front 6 What pathways can degrade proteins? | back 6 Cytosolic ATP dependent and Lysosomal |
front 7 What is a lysosome? | back 7 membrane-enclosed organelle that contain the digestive enzymes: lipases, nucleases and proteases. |
front 8 What is the function of a lysosome? | back 8 the digestive system of the cell, breaks down waste materials, cellular debris, and foreign particles in the cell using digestive enzymes |
front 9 What is the term for the cellular process that breaks down and recycles olf, damaged, or abnormal cell parts? | back 9 Autophagy |
front 10 What is the process of autophagy? | back 10 a process where vesicles called autophagosomes engulf small amounts of cytoplasm or specific organelles using the endoplasmic reticulum |
front 11 what occurs when vesicles fuse with lysosomes? | back 11 the release of the lysosomal hydrolytic enzymes causing degradation of macromolecules |
front 12 LC3-I and LC3-II does what for autophagosomes | back 12 decides and regulates |
front 13 When you have more LC3-II you will have more | back 13 autophagosomes |
front 14 What is a autolysosome? | back 14 a compartment that is created when an autophagosome fuses with a lysosome |
front 15 Lysosomes can selectivley degrade- | back 15 cytosolic proteins |
front 16 The target proteins of lysosomes are usallly proteins with | back 16 long half lives |
front 17 The function of dispensible proteins is - | back 17 to be sacrifies to make amino acids and energy to support basic metabolic reactions |
front 18 The ATP dependent pathway involves the protein ______________ | back 18 ubiquitin (highly conserved protein containing 76 amino acids) |
front 19 What is the function of ubiquitin in the ATP dependent pathway? | back 19 it tags proteins that are destined for destruction via. a covalent attatchment |
front 20 What are the mechanisms for recognition? | back 20 Half-life of proteins, recogition of phsophorylated substrates, recogition of ancillary porteins bound to the substrate, recogition of mutated abnormal protein. |
front 21 The half-life of proteins correlates with what? | back 21 amino-terminal residue |
front 22 Proteins with N-terminal Met, Ser, Ala, Thr, Val, or Gly have half lives greater than? | back 22 20 hours |
front 23 Proteins with the N-terminal Phe, Leu, Asp, Lys, or Arg have half-lives of ? | back 23 3 mins or less. |
front 24 Proteins rich in Pro (P), Glu (E), Ser (S), and Thr (T) are more ____________ degraded than other proteins | back 24 rapidly |
front 25 Ubiquitin is ____________ linked to proteins through the ATP dependent pathway involving enzymes ___ , ____ , ____. | back 25 covalently, E1, E2, E3 |
front 26 Ubiquitination of proteins results in what? | back 26 the linking the carboxy-terminal glycine residue in ubiquitin to a lysine residue in the protein to be degraded |
front 27 What is the function of the E1 enzyme? | back 27 acitivates ubiquitin, to bind to it |
front 28 What is the function of the E2 enzyme? | back 28 conjugates ubiquitin |
front 29 The function of E3 enzyme? | back 29 ubitquitin ligase, promotes the transfer of ubiquitin from E2 to the lysine residue of the protein recognized by the E3 as being slated for degradation. |
front 30 What is the 26S proteosome? | back 30 a large complex of proteins that structurally resembles a large cylinder that is covered on both ends. |
front 31 What is the central 20S core particle? | back 31 is a barrel shaped formed of 4 rings, outer rings formed of 7 alpha rings and inner rings formed of 7 beta rings |
front 32 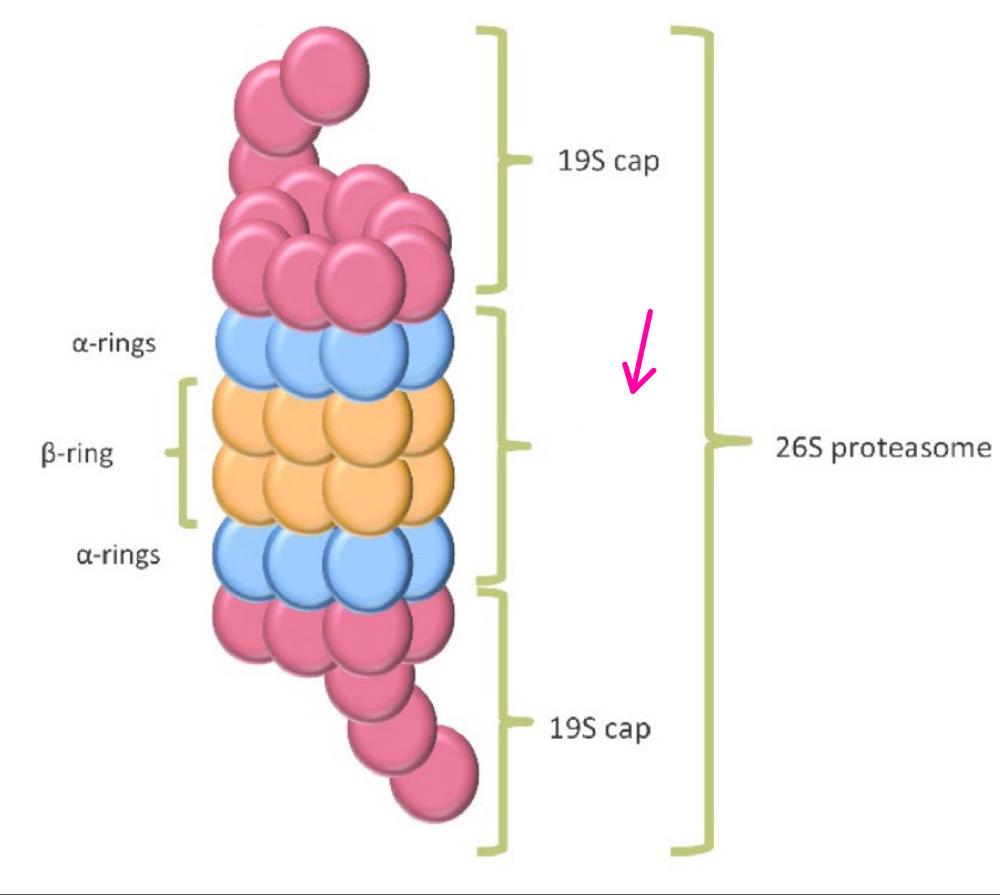 What part of the proteosome is this? | back 32 The central 20S core particle |
front 33 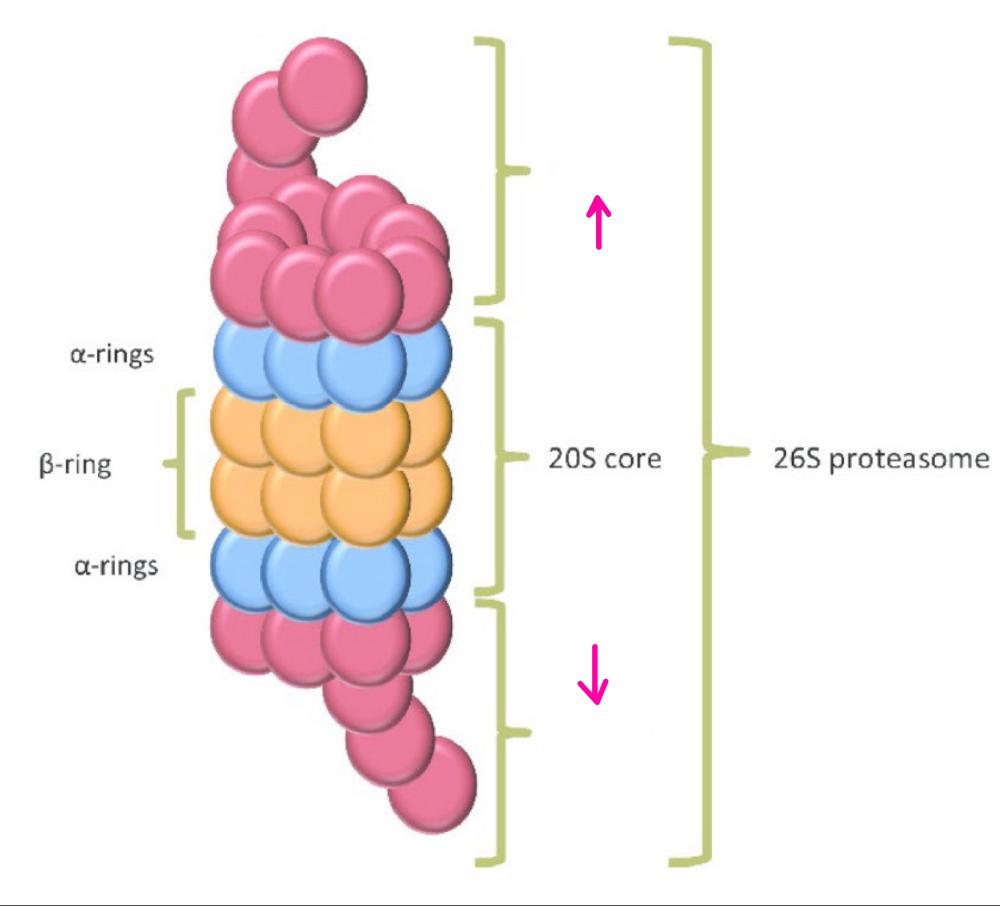 What part(s) of the proteosome is this? | back 33 The 19S regulatory particle caps |
front 34 Why are the 19S regulatory caps important? | back 34 they are important for recogition and binding of polyubiquinated proteins, removal of ubiquitin, unfolding of protein substrate, and translocation into the central core |
front 35 How many ubiquitin are needed to be recoginzed by proteosome? | back 35 a minimum of 4 |
front 36 Degradation of proteins in the proteosomes are faciliated by ________ and hydrolyzed by ________ | back 36 19S particles, central 20S core |
front 37 What is bortezomib? | back 37 the 1st therapeutic proteosome inhibitor to be tested in humans. |
front 38 What is the function of bortezomib? | back 38 a peptide that binds in the catalytic site of the 26S proteosome and inhibits the degradtionof proteins |