Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chemistry Flashcards 1.0
front 1 Structure of diamond | back 1 A tetrahedral shape, with each carbon atom bonded to 4 other carbons with strong covalent bonds. |
front 2 Graphite structure | back 2 Layers of carbon atoms in a hexagon arrangement with weak intermolecular forces between them. Each carbon atom is bonded to 3 other carbons with covalent bonds with one free (delocalised) electron per carbon atom. |
front 3 The structure of silicon dioxide | back 3 A tetrahedral structure where each silicon atom is bonded to 4 oxygen atoms, and each oxygen atom is bonded to 2 silicon atoms. |
front 4 Why diamond is a cutting tool. | back 4 Rigid three-dimensional structure with many strong covalent bonds making it very hard. |
front 5 Similarities od diamond and sio2 | back 5 High melting and boiling points as strong covalent bonds, very hard as three-dimensional structure + strong bonds, no delocalised electrons so can't conduct |
front 6 Metals are malleable and ductile because... | back 6 The layers of ions can slide over each other but are still held together by the delocalised electrons so can be bent into shape (malleable) and stretched out (ductile). |
front 7 Ionic bond meaning | back 7 An electrostatic force between oppositely charged ions. |
front 8 Covalent bond meaning | back 8 When a pair of electrons is shared between two atoms to gain full outer shells of electrons. |
front 9 precipitation reaction | back 9 Two soluble reactants form an insoluble product and a soluble product. |
front 10 Molecular formula | back 10 The number and type of different atoms in one molecule. |
front 11 Relative atomic mass | back 11 The average mass of the isotopes of an element compared the mass of an atom of carbon-12. |
front 12 Avogadro constant (number of particles one mole contains) | back 12 6.02 × 10(23) |
front 13 Acid with metal | back 13 Salt and hydrogen |
front 14 Acid with base | back 14 Neutralise to salt and water |
front 15 Acid with carbonates | back 15 Salt, CO2, and water. |
front 16 Acid properties | back 16 Sour, corrosive, below 7 PH, proton donor. Presence of H+ ions makes it acidic. |
front 17 Alkali and ammonium salt | back 17 Water, salt and ammonia gas. |
front 18 Symbol equation for to show HCl is a strong acid | back 18 HCl (aq) → H+(aq) + Cl –(aq) |
front 19 Symbol equation to show ethnic acid is a week acid | back 19 CH3COOH(aq) ⇌ H+(aq) + CH3COO–(aq) |
front 20 Neutral oxides. | back 20 carbon monoxide and nitrate oxides. |
front 21 Preparing salt by titration. | back 21 Using pipette measure alkali into conical flask adding methyl orange. Add acid to burette noting starting volume. Slowly add acid to alkali until turns red. Record finishing volume and calculate how much acid was added. Add this amount of acid with same amount of alkali as first measured out without indicator. Form saturated solution by partially evaporating, then crystallise and dry. |
front 22 Preparing salt with solid metal, base or carbonate. | back 22 Heat dilute acid in beaker over a Bunsen burner. Add insoluble metal, base or carbonate until in excess. Filter then evaporate into saturated solution. Leave to crystallise and dry crystals. |
front 23 Hydrated substance | back 23 A substance chemically combined with water. |
front 24 The term water of crystallisation. | back 24 The water molecules present in hydrated crystals. |
front 25 Preparation of insoluble salts | back 25 Precipitation reaction: Dissolve soluble salts in water and mix together using a stirring rod in a beaker. Filter and wash filtrate with water to remove traces of other solutions. Dry. |
front 26 Appearance of bromine, iodine and chlorine. | back 26 Bromine - red-brown liquid. Chlorine - Yellow-green gas Iodine - Grey black solid. |
front 27 Metal and steam reaction | back 27 Form solid metal oxide and hydrogen gas. |
front 28 Aluminium in overhead cables | back 28 Good conductivity and low density |
front 29 Copper in overhead cables | back 29 Good conductivity and ductility. |
front 30 Aluminium in food containers | back 30 Resistant to corrosion. |
front 31 Brass made out of... | back 31 Mixture of copper and zinc. |
front 32 Stainless steel made of... | back 32 Mixture of iron and other elements such as chromium, nickel and carbon. |
front 33 Stainless steel is used for cutlery because... | back 33 It's hardness and resistance to corrosion. |
front 34 Brass is used for instruments because... | back 34 Its resistance to corrosion and good malleability. |
front 35 State the order of reactivity. | back 35 Potassium, sodium, calcium, magnesium, aluminium, carbon, zinc, iron, hydrogen, copper, silver, gold. |
front 36 Reaction of calcium with water. | back 36 Fizzing, disappears, no flame, white precipitate formed. |
front 37 Magnesium with steam reaction | back 37 Burns in steam to form white magnesium oxide and hydrogen. |
front 38 Magnesium and hydrochloric acid. | back 38 Reacts rapidly and vigorously producing magnesium chloride and hydrogen gas. |
front 39 Zinc and hydrochloric acid. | back 39 Also reacts but at a slower rate than magnesium, producing zinc chloride and hydrogen gas. |
front 40 Iron and hydrochloric acid. | back 40 Reacts slowly forming iron(II) chloride and hydrogen gas. |
front 41 Copper and hydrochloric acid. | back 41 Barely reacts. |
front 42 Ionic half equations for extraction of aluminium | back 42 Al3+ + 3e- → Al 2O2- → O2 + 4e- |
front 43 Water from natural sources may contain substances, including... and which are helpful and not. | back 43 Dissolved oxygen, metal compounds, plastics, sewage, harmful microbes, nitrates from fertilisers, phosphates from fertilisers and detergents. Dissolved oxygen for aquatic life, some metal compounds provide essential minerals for life but others are toxic, and nitrates and phosphates lead to deoxygenation of water and damage to aquatic life. |
front 44 Steps of filtration | back 44 Sedimentation and filtration, use of carbon to remove tastes and odours, chlorination to kill microbes. |
front 45 How is sulphur dioxide formed | back 45 From the combustion of fossil fuels which contain sulfur compounds. |
front 46 Catalytic converters reaction. | back 46 2CO + 2NO → 2CO2 + N2 |
front 47 State the symbol equation for photosynthesis. | back 47 6CO2 + 6H2O → C6H12O6 + 6O2 |
front 48 What is kerosene / parrafin used for? | back 48 Jet fuel |
front 49 What fuel oil fraction is used for. | back 49 Fuel for ships and home heating systems. |
front 50 What the lubricating oil fraction is used for. | back 50 Lubricants, waxes and polishes. |
front 51 Reactivity of alkanes | back 51 Generally unreactive, except in terms of combustion and substitution by chlorine. |
front 52 Describe the manufacture of alkenes. | back 52 Cracking of larger alkane molecules using a high temperature and a catalyst |
front 53 Describe the reasons for the cracking of larger alkane molecules. | back 53 Matches supply with demand, and alkenes are useful as feedstock in the photochemical industry. |
front 54 Describe the properties of alkenes in terms of addition reactions with hydrogen with structural formula. | back 54 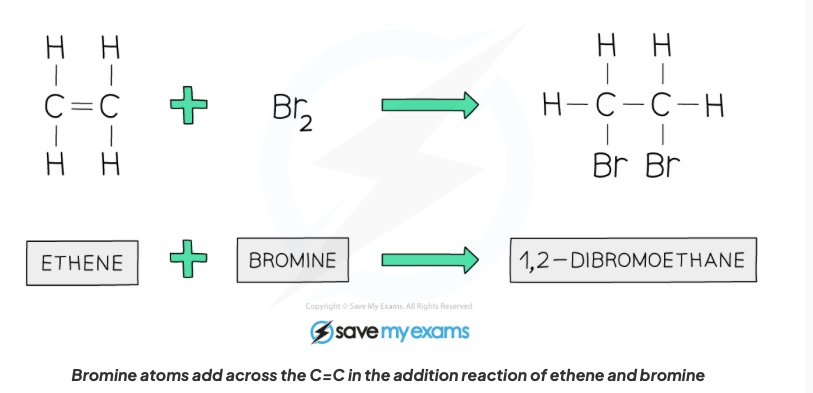 An alkane is formed, a hydrogen atom gets added to each bond where the C=C bond is broken. |
front 55 Describe the properties of alkenes in terms of addition reactions with hydrogen in the presence of a nickel catalyst, with structural formula. | back 55 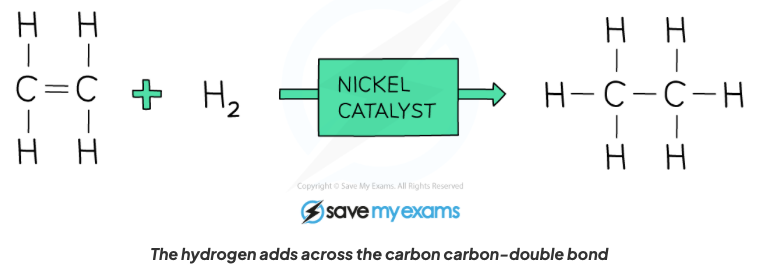 An alkane is formed, a hydrogen atom gets added to each bond where the C=C bond is broken. |
front 56 Describe the properties of alkenes in terms of addition reactions with steam in the presence of an acid catalyst. | back 56 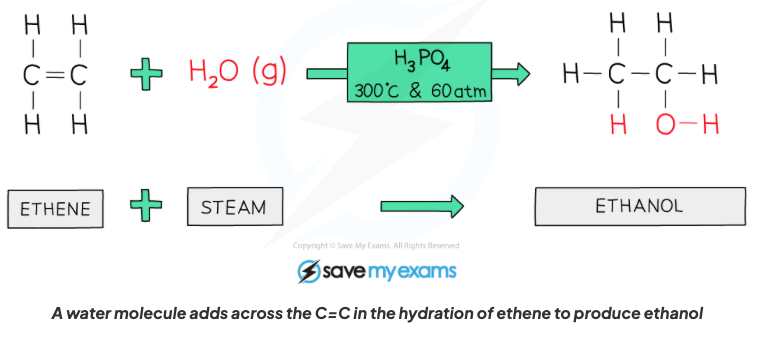 An alcohol is formed, it is also a hydration reaction as a water molecule is being added, C=C bond breaks and one water molecule is added to turn eg (ethene into ethanol or propane into propanol and so on). |
front 57 Describe the manufacture of ethanol by fermentation. | back 57 Fermentation of aqueous glucose at 25–35 °C in the presence of yeast and in the absence of oxygen. |
front 58 Describe the manufacture of ethanol by steam. | back 58 Catalytic addition of steam to ethene, at 300°C and 60 atm in the presence of an acid catalyst. |
front 59 Describe the combustion of ethanol. | back 59  Ethanol burns with an almost invisible blue flame, burns cleanly, and without strong odours. Alcohols undergo combustion to form carbon dioxide and water. |
front 60 Describe the advantages and disadvantages of the manufacture of ethanol by: (a) fermentation | back 60 Fermentation: simple equipment and low temperatures required which saves money, and uses renewable recourses. It however produces greenhouse gas co2, is very slow and produces a dilute solution that needs further processing, also it is made in batches. Hydration: Complex set up, uses non-renewable recourses but produces no greenhouse gases, is a fast continues process that produces pure ethanol, but high temperatures and pressures required increasing the energy input and cost. |
front 61 Products of ethanoic acid and: a. Metal 2CH3COOH + Mg → ? b. base CH₃COOH + NaOH → ? c. Carbonates 2CH₃COOH + Na₂CO₃ → ? | back 61 The name is the name of the metal before ethanoate. a. (CH₃COO)₂Mg + H₂ b. CH₃COONa + H₂O c. 2CH₃COONa + CO₂ + H₂O |
front 62 Describe the formation of ethanoic acid by the oxidation of ethanol with acidified aqueous potassium manganate(VII). | back 62 Heating ethanol and acidified aqueous potassium manganate(VII) in the presence of an acid in a vessel with a condenser attached to stop the alcohol with its low boiling point escaping. goes from colourless to purple. CH3CH2OH (aq) + 2[O] → CH3COOH (aq) + H2O (l) |
front 63 Describe the formation of ethanoic acid by the oxidation of ethanol by bacterial oxidation during vinegar production. | back 63 Acetobacter bacteria uses atmospheric oxygen from air to oxidise ethanol in wine, producing a weak solution of ethanoic acid (vinegar) this is what makes one have an acidic vinegary taste after it has been opened for a while. |
front 64 Ester reaction (ethanoic acid and ethanol) | back 64 Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate and water. |
front 65 Describe how the properties of plastics have implications for their disposal. | back 65 Many polymers are chemically unreactive so they are non-biodegradable and fill up land fill space and oceans. Many also create toxic gas if burned so incineration is very bad. |
front 66 What a polyamide is made from and its link. | back 66 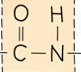 A dicarboxylic acid and a diamine. |
front 67 What a polyester is made of and its link. | back 67 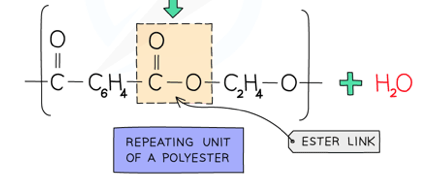 From a dicarboxylic acid and a diol. |
front 68 draw the structure of nylon, a polyamide. | back 68 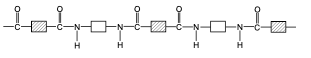 |
front 69 draw the structure of PET, a polyester | back 69 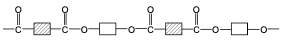 |
front 70 General structure of amino acid monomers. | back 70 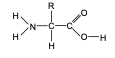 where R represents different types of side chain |
front 71 Draw the structure of proteins. | back 71 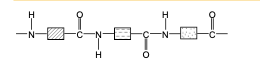 |
front 72 Test for nitrate, NO3 – ion. | back 72 Reduction with aluminium foil and aqueous sodium hydroxide and then testing for ammonia gas. |
front 73 Test for sulfate, SO4 2- | back 73 Acidifying with dilute nitric acid and then adding aqueous barium nitrate. |
front 74 Test for sulfite, SO3 2- | back 74 Reaction with acidified aqueous potassium manganate(VII). |
front 75 Identify test and result for each. (a) aluminium, Al 3+ (b) ammonium, NH4+ (c) calcium, Ca2+ (d) chromium(III), Cr3+ (e) copper(II), Cu2+ (f) iron(II), Fe2+ (g) iron(III), Fe3+ (h) zinc, Zn2+ | back 75 a. Dilute NaOH or ammonia, white precipitate (soluble in excess NaOH, insoluble in excess ammonia) b. Dilute warm NaOH, ammonia produced c. Dilute NaOH, white precipitate (insoluble in excess NaOH) d. Dilute NaOH or ammonia solution, grey-green precipitate (soluble in excess NaOH, insoluble in excess ammonia) e. Dilute NaOH or ammonia solution, light blue precipitate (insoluble in excess NaOH, soluble in excess ammonia) f. Dilute NaOH or ammonia solution, green precipitate (insoluble in excess both) g. Dilute NaOH or ammonia solution, red-brown precipitate (insoluble in excess both) h. Dilute NaOH or ammonia solution, white precipitate (soluble in excess both) |