Structure of diamond
A tetrahedral shape, with each carbon atom bonded to 4 other carbons with strong covalent bonds.
Graphite structure
Layers of carbon atoms in a hexagon arrangement with weak intermolecular forces between them. Each carbon atom is bonded to 3 other carbons with covalent bonds with one free (delocalised) electron per carbon atom.
The structure of silicon dioxide
A tetrahedral structure where each silicon atom is bonded to 4 oxygen atoms, and each oxygen atom is bonded to 2 silicon atoms.
Why diamond is a cutting tool.
Rigid three-dimensional structure with many strong covalent bonds making it very hard.
Similarities od diamond and sio2
High melting and boiling points as strong covalent bonds, very hard as three-dimensional structure + strong bonds, no delocalised electrons so can't conduct
Metals are malleable and ductile because...
The layers of ions can slide over each other but are still held together by the delocalised electrons so can be bent into shape (malleable) and stretched out (ductile).
Ionic bond meaning
An electrostatic force between oppositely charged ions.
Covalent bond meaning
When a pair of electrons is shared between two atoms to gain full outer shells of electrons.
precipitation reaction
Two soluble reactants form an insoluble product and a soluble product.
Molecular formula
The number and type of different atoms in one molecule.
Relative atomic mass
The average mass of the isotopes of an element compared the mass of an atom of carbon-12.
Avogadro constant (number of particles one mole contains)
6.02 × 10(23)
Acid with metal
Salt and hydrogen
Acid with base
Neutralise to salt and water
Acid with carbonates
Salt, CO2, and water.
Acid properties
Sour, corrosive, below 7 PH, proton donor. Presence of H+ ions makes it acidic.
Alkali and ammonium salt
Water, salt and ammonia gas.
Symbol equation for to show HCl is a strong acid
HCl (aq) → H+(aq) + Cl –(aq)
Symbol equation to show ethnic acid is a week acid
CH3COOH(aq) ⇌ H+(aq) + CH3COO–(aq)
Neutral oxides.
carbon monoxide and nitrate oxides.
Preparing salt by titration.
Using pipette measure alkali into conical flask adding methyl orange. Add acid to burette noting starting volume. Slowly add acid to alkali until turns red. Record finishing volume and calculate how much acid was added. Add this amount of acid with same amount of alkali as first measured out without indicator. Form saturated solution by partially evaporating, then crystallise and dry.
Preparing salt with solid metal, base or carbonate.
Heat dilute acid in beaker over a Bunsen burner. Add insoluble metal, base or carbonate until in excess. Filter then evaporate into saturated solution. Leave to crystallise and dry crystals.
Hydrated substance
A substance chemically combined with water.
The term water of crystallisation.
The water molecules present in hydrated crystals.
Preparation of insoluble salts
Precipitation reaction: Dissolve soluble salts in water and mix together using a stirring rod in a beaker. Filter and wash filtrate with water to remove traces of other solutions. Dry.
Appearance of bromine, iodine and chlorine.
Bromine - red-brown liquid.
Chlorine - Yellow-green gas
Iodine - Grey black solid.
Metal and steam reaction
Form solid metal oxide and hydrogen gas.
Aluminium in overhead cables
Good conductivity and low density
Copper in overhead cables
Good conductivity and ductility.
Aluminium in food containers
Resistant to corrosion.
Brass made out of...
Mixture of copper and zinc.
Stainless steel made of...
Mixture of iron and other elements such as chromium, nickel and carbon.
Stainless steel is used for cutlery because...
It's hardness and resistance to corrosion.
Brass is used for instruments because...
Its resistance to corrosion and good malleability.
State the order of reactivity.
Potassium, sodium, calcium, magnesium, aluminium, carbon, zinc, iron, hydrogen, copper, silver, gold.
Reaction of calcium with water.
Fizzing, disappears, no flame, white precipitate formed.
Magnesium with steam reaction
Burns in steam to form white magnesium oxide and hydrogen.
Magnesium and hydrochloric acid.
Reacts rapidly and vigorously producing magnesium chloride and hydrogen gas.
Zinc and hydrochloric acid.
Also reacts but at a slower rate than magnesium, producing zinc chloride and hydrogen gas.
Iron and hydrochloric acid.
Reacts slowly forming iron(II) chloride and hydrogen gas.
Copper and hydrochloric acid.
Barely reacts.
Ionic half equations for extraction of aluminium
Al3+ + 3e- → Al
2O2- → O2 + 4e-
Water from natural sources may contain substances, including...
and which are helpful and not.
Dissolved oxygen, metal compounds, plastics, sewage, harmful microbes, nitrates from fertilisers, phosphates from fertilisers and detergents.
Dissolved oxygen for aquatic life, some metal compounds provide essential minerals for life but others are toxic, and nitrates and phosphates lead to deoxygenation of water and damage to aquatic life.
Steps of filtration
Sedimentation and filtration, use of carbon to remove tastes and odours, chlorination to kill microbes.
How is sulphur dioxide formed
From the combustion of fossil fuels which contain sulfur compounds.
Catalytic converters reaction.
2CO + 2NO → 2CO2 + N2
State the symbol equation for photosynthesis.
6CO2 + 6H2O → C6H12O6 + 6O2
What is kerosene / parrafin used for?
Jet fuel
What fuel oil fraction is used for.
Fuel for ships and home heating systems.
What the lubricating oil fraction is used for.
Lubricants, waxes and polishes.
Reactivity of alkanes
Generally unreactive, except in terms of combustion and substitution by chlorine.
Describe the manufacture of alkenes.
Cracking of larger alkane molecules using a high temperature and a catalyst
Describe the reasons for the cracking of larger alkane molecules.
Matches supply with demand, and alkenes are useful as feedstock in the photochemical industry.
Describe the properties of alkenes in terms of addition reactions with hydrogen with structural formula.
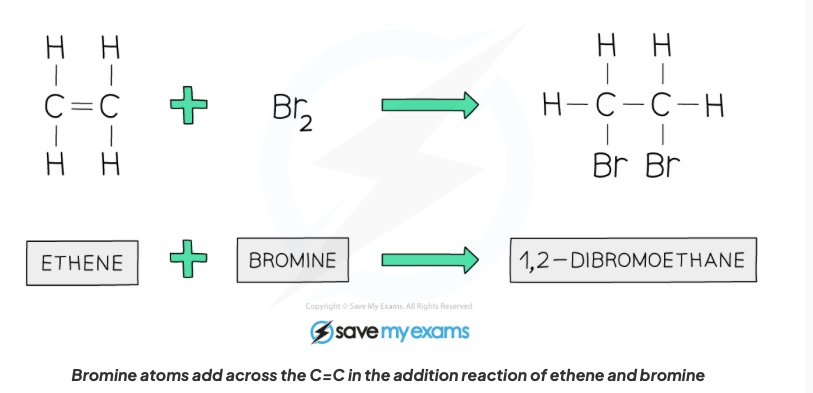
An alkane is formed, a hydrogen atom gets added to each bond where the C=C bond is broken.
Describe the properties of alkenes in terms of addition reactions with hydrogen in the presence of a nickel catalyst, with structural formula.
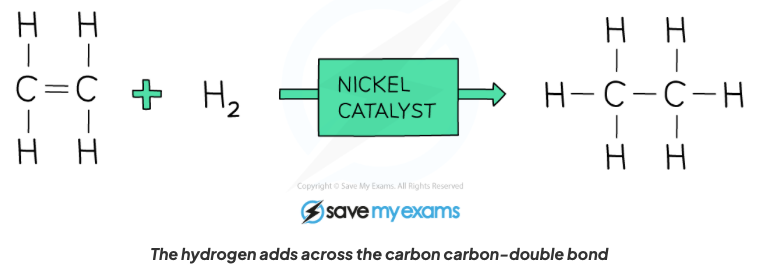
An alkane is formed, a hydrogen atom gets added to each bond where the C=C bond is broken.
Describe the properties of alkenes in terms of addition reactions with steam in the presence of an acid catalyst.
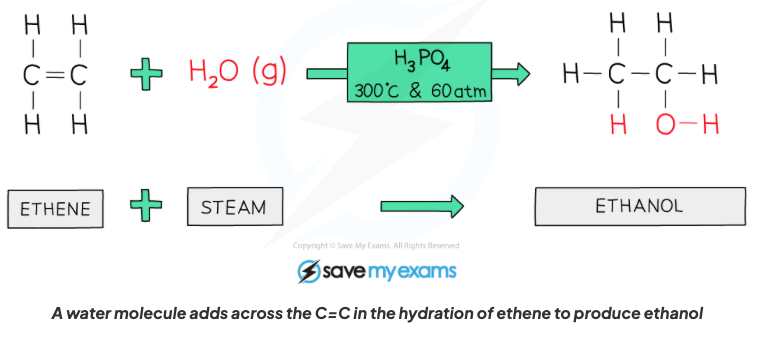
An alcohol is formed, it is also a hydration reaction as a water molecule is being added, C=C bond breaks and one water molecule is added to turn eg (ethene into ethanol or propane into propanol and so on).
Describe the manufacture of ethanol by fermentation.
Fermentation of aqueous glucose at 25–35 °C in the presence of yeast and in the absence of oxygen.
Describe the manufacture of ethanol by steam.
Catalytic addition of steam to ethene, at 300°C and 60 atm in the presence of an acid catalyst.
Describe the combustion of ethanol.

Ethanol burns with an almost invisible blue flame, burns cleanly, and without strong odours.
Alcohols undergo combustion to form carbon dioxide and water.
Describe the advantages and disadvantages of the manufacture of ethanol by:
(a) fermentation
(b) catalytic addition of steam to ethene
Fermentation: simple equipment and low temperatures required which saves money, and uses renewable recourses. It however produces greenhouse gas co2, is very slow and produces a dilute solution that needs further processing, also it is made in batches.
Hydration: Complex set up, uses non-renewable recourses but produces no greenhouse gases, is a fast continues process that produces pure ethanol, but high temperatures and pressures required increasing the energy input and cost.
Products of ethanoic acid and:
a. Metal 2CH3COOH + Mg → ?
b. base CH₃COOH + NaOH → ?
c. Carbonates 2CH₃COOH + Na₂CO₃ → ?
The name is the name of the metal before ethanoate.
a. (CH₃COO)₂Mg + H₂
b. CH₃COONa + H₂O
c. 2CH₃COONa + CO₂ + H₂O
Describe the formation of ethanoic acid by the oxidation of ethanol with acidified aqueous potassium manganate(VII).
Heating ethanol and acidified aqueous potassium manganate(VII) in the presence of an acid in a vessel with a condenser attached to stop the alcohol with its low boiling point escaping. goes from colourless to purple.
CH3CH2OH (aq) + 2[O] → CH3COOH (aq) + H2O (l)
Describe the formation of ethanoic acid by the oxidation of ethanol by bacterial oxidation during vinegar production.
Acetobacter bacteria uses atmospheric oxygen from air to oxidise ethanol in wine, producing a weak solution of ethanoic acid (vinegar) this is what makes one have an acidic vinegary taste after it has been opened for a while.
Ester reaction (ethanoic acid and ethanol)
Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate and water.
Describe how the properties of plastics have implications for their disposal.
Many polymers are chemically unreactive so they are non-biodegradable and fill up land fill space and oceans. Many also create toxic gas if burned so incineration is very bad.
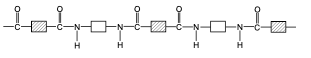
What a polyamide is made from and its link.
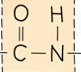
A dicarboxylic acid and a diamine.
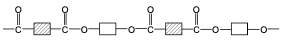
What a polyester is made of and its link.
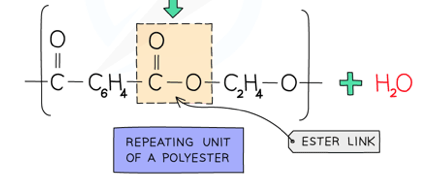
From a dicarboxylic acid and a diol.
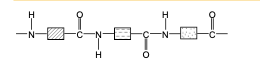
draw the structure of nylon, a polyamide.
draw the structure of PET, a polyester
General structure of amino acid monomers.
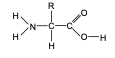
where R represents different types of side chain
Draw the structure of proteins.
Test for nitrate, NO3 – ion.
Reduction with aluminium foil and aqueous sodium hydroxide and then testing for ammonia gas.
Test for sulfate, SO4 2-
Acidifying with dilute nitric acid and then adding aqueous barium nitrate.
Test for sulfite, SO3 2-
Reaction with acidified aqueous potassium manganate(VII).
Identify test and result for each.
(a) aluminium, Al 3+
(b) ammonium, NH4+
(c) calcium, Ca2+
(d) chromium(III), Cr3+
(e) copper(II), Cu2+
(f) iron(II), Fe2+
(g) iron(III), Fe3+
(h) zinc, Zn2+
a. Dilute NaOH or ammonia, white precipitate (soluble in excess NaOH, insoluble in excess ammonia)
b. Dilute warm NaOH, ammonia produced
c. Dilute NaOH, white precipitate (insoluble in excess NaOH)
d. Dilute NaOH or ammonia solution, grey-green precipitate (soluble in excess NaOH, insoluble in excess ammonia)
e. Dilute NaOH or ammonia solution, light blue precipitate (insoluble in excess NaOH, soluble in excess ammonia)
f. Dilute NaOH or ammonia solution, green precipitate (insoluble in excess both)
g. Dilute NaOH or ammonia solution, red-brown precipitate (insoluble in excess both)
h. Dilute NaOH or ammonia solution, white precipitate (soluble in excess both)