Charles Darwin proposed a mechanism for descent with modification
that stated that organisms of a particular species are adapted to
their environment when they possess
A) non-heritable traits that
enhanced their survival in the local environment
B)non-inheritable traits that enhance their survival and
reproductive success in the local environment
C) heritable traits
that enhance their survival and reproductive success in the local
environment
D) heritable traits that decrease their survival and
reproductive success in the local environment
C) heritable traits that enhance their survival and reproductive success in the local environment
When an electron moves from the 4th principal energy level to the 3rd
principal energy level, energy is:
A)released
B)destroyed
C)created
D)absorbed
A) released
The entire "library" of genetic instructions that an
organism inherits is called its
A)gene
B)bioinformatics
C)genome
D)proteome
C)genome
Water molecules are attracted to one another by...
A)ionic
bonds
B)no polar covalent bonds
C)hydrophobic
interactions
D)hydrogen bonds
D)hydrogen bond
A covalent bond is one in which...
A)protons and neutrons are
shared by two atoms so as to satisfy the requirements of both atoms
B)electrons are removed from one atom and transferred to another
atom so that the two atoms become oppositely charged
C)outer
shell elections of two atoms are shared so as to satisfactorily fill
their respective orbitals
D)outer shell electrons of one atom are
transferred to fill the inner electron shell of another atom
C)outer shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
Which of the following effects can occur because of the high surface
tension of water?
A)lake cannot freeze solid in winter, despite
low temperatures.
B)a raft spider can walk across the surface of
a small pond
C)organisms can resist temperature changes, although
they give off heat due to chemical reactions
D)sweat can
evaporate from the skin, helping to keep people from overheating.
B)a raft spider can walk across the surface of a small pond
Bonds between two atoms that are equally electronegative
are
A)polar covalent bonds
B)hydrogen bonds
C)ionic
bonds
D)nonpolar covalent bonds
D)nonpolar covalent bonds
In what type of chemical bond are electrons transferred from metal to
non metal?
A)hydrogen
B)ionic
C)van der waals
D)covalent
B)ionic
To understand the chemical basis of inheritance, we must understand
the molecular structure of DNA. This is an example of the application
of which concept to the study of
biology?
A)evolution
B)Feedback
regulation
C)reductionism
D)emergent properties
C)reductionism
Water has many exceptional and useful properties. Which is the rarest property among compounds?
A)water is a solvent
B)water has a high heat capacity
C)solid water is less dense than liquid warer
D)water has surface tension
C)solid water is less dense than liquid water
In a single molecule of water, two hydrogen atoms are bonded to a
single oxygen atom by
A)hydrogen bonds
B)ionic
bonds
C)no polar covalent bonds
D)polar covalent bonds
D)polar covalent bond
When are atoms most stable?
A)when they have the fewest possible
valence electrons
B)when they have the maximum number of unpaired
electrons
C)when all of the electron r vitals in the valence
shell are filled
D)when all electrons are paired
C)when all the electron orbitals in the valence shell are filled
Prokaryotes are classified as belonging to two different domains.
What are the domains?
A)bacteria and Protista
B)bacteria and
archaea
C)archaea and monetary
D)bacteria and eukarya
B)bacteria and archea
What property of water allows for transport of water against gravity in plants?
A)kinetic energy
B)cohesion
C)surface tension
D)specific heat
B)cohesion
Which two particles have approximately the same mass?
A)electron and neutron
B)neutron and neutrino
C)proton and neutron
D)proton and electron
C)proton and neutron
Knowing the atomic mass of an element allows inferences about which of the following?
A)the number of protons plus neutrons in the element
B)the number of electrons in the element
C)the number of protons plus electrons in the elemenr
D)the hunger of protons in the element
A)the number of protonsvolus neutrons in the element
A localized group of organisms that belong to the same species is called a _______.
A)family
B)community
C)ecosystem
D)population
D)population
Which component of a solution is responsible for dissolving?
A)unsaturated
B)solute
C)solvent
D)saturated
C)solvent
Which of the following correctly describes a reaction that has reached chemical equilibrium?
A)all of the reactants have been converted to the products of the reaction
B)the rate of the forward reaction is equal to the rate of the reverse reaction.
C)all of the products have been converted to the reactants of the reaction,
D)both the forward and the reverse reactions have stopped, with no net effect on the concentration of the reactants and the products.
B)the rate of the forward reaction is equal to the rate of the reverse reaction.
In comparison to eukaryotes, prokaryotes ______.
A)do no have membranes
B)are smaller
C)are larger
D)are more structurally complex
B)are smaller
Which of the following are compounds?
A)H2O and O2
B)H2O and CH4, but not O2
C)H2O, O2, and CH4
D)O2 and CH4
B)H2O and CH4, but not O2

Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely ______.
A)positively charged
B)nonpolar
C)negatively charged
D)without charge
A)positively charged
When a person gets dehydrated while exercising on a hot day, their pituitary gland releases ADH, a hormone that signals the kidneys to retain more water. This is an example of...
A)positive feedback regulation
B)Emergent properties
C)negative feedback regulation
D)Chemical cycling
C)negative feedback regulation
You need to write down information about a molecule, but need to indicate only the type and number of atoms it contains. Which representation would work best?
A)structural formula
B)ball-and-stick model
C)space-filling model
D)molecular formula
D) molecular formula
Which of the following is property of liquid water? Liquid water _____.
A)is non polar
B)has a heat of vaporization that is higher than that for mos tother substances
C)has a specific heat that is lower than that for most other substances
D)is less dense than ice
B)has a heat of vaporization that is higher than that for most other substances
What results from an unequal sharing of electrons between atons?
A)an ionic bond
B)a nonpolar covalent bond
C)a polar covalent bond
D)a hydrophobic interaction
C)a polar covalent bond
Which of the following solution would require the addition of the greatest amount of base to bring the solution to neutral pH?
A)gastric juice at pH 2
B)vinegar at pH 3
C)household bleach at pH 12
D) black coffee at pH 5
A) gastric joice at pH 2
From its atomic number of 15, it is possible to predict that the phosphorus atom has ____.
A)5 neutrons, 5 protons, and 5 electrons
B)15 neutrons and 15 protons
C)8 electrons in its outermost electron shell
D)15 protons and 15 electrons
D)15 protons and 15 electrons
About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
A)oxygen, hydrogen, calcium, nitrogen
B)carbon, sodium, hydrogen, nitrogen
C)carbon, oxygen, phosphorus, hydrogen
D)carbon, hydrogen, nitrogen, oxygen
D)carbon, hydrogen, nitrogen, oxygen
A solution with a pH of 5 has how many more protons in it than a solution with a pH of 7?
A)5 times
B)1000 times
C)100 times
D)10 times
C)100 times
Hydrophobic substance such as vegetable oil are ____.
A)nonpolar substances that repel water molecules
B)non polar substances that have an attraction for water molecules
C)polar substances that repel water molecules
D)polar substances that have an affinity for water
A)nonpolar substance sthat repel water molecules
What type of elements are required by an organism in minute quanitites?
A)trace
B)dietary
C)essential
A)trace
The addition of what compound to the water supply is most responsible for ocean solidification?
A)O2
B)NaOH
C)HCl
D)CO2
D)CO2
How many electrons are involved in a single covalent bond?
A)one
B)iodine
C)two
D)three
E)four
C)two
Which of these provide evidence of a common ancestry of all
life?
A)near universality of the genetic code
B)structure of chloroplasts
C)structure of the nucleus
D)structure of cilia
A)near universality of the genetic code
Which of the following statements is true about buffer solutions?
A)they fluctuate in pH when either acids or bases are added to them
B)they maintain a constant pH when bases are added to them but not when acids are added to them.
C)they maintain a constant pH when acids are added to them but not when bases are added to them
D)they maintain a relatively constant pH when either acids or bases are added to them
D)they maintain a relatively constant pH when either acids or bases are added to them
Isotopes of a given element have the same number of what?
A)protons
B)orbitals
C)electrons
D)neutrons
A)protons
In the organism Culex pipiens - Culex refers to the organism's
A)kingdom
B)species
C)family
D)genus
D)genus
A substance that contains more hydroxide ions than hydronium ions is termed?
A)acidic
B)neutral
C)saturated
D)basic
D)basic
Which of the following types of cells utilize deoxyribonucleic acid (DNA) as their genetic material but do not have their DNA encased within a nuclear envelope?
A)plant
B)animal
C)fungi
D)archaean
D)archaean
For an ecosystem to be self-sustaining it requires a constant flow of energy into the system and
A)interactions with the environment
B)consumers
C)feedback regulation
D)cycling of nutrients
D)cycling of nutrients
Water molecules can form hydrogen bonds with ____.
A)chloride ions
B)oxygen gas O2 molecules
C)compounds that have polar covalent bonds
D)oils
C)compounds that have polar covalent bonds
Acids release which type of ion in solution
A)hydration
B)hydroxide
C)water
D)hydrogen
D)hydrogen
Cell are _____.
A)only found in pairs because single cells cannot exist independently
B)characteristic of prokaryotic and eukaryotic organisms
C)characteristics of eukaryotic but not prokaryotic organisms
D)limited in size to 200 and 50 micrometers in diameter
B)characteristic of prokaryotic and eukaryotic organisms
Which subatomic particle is responsible for the activity of the aotm?
A)neutron
B)electron
C)proton
D)alpha particle
B)electron
Which of the following best summarizes the relationship between dehydration reaction and hydrolysis?
A)Dehydration reactions and hydrolysis reactions assemble polymers from monomers.
B)Dehydration reactions assemble polymers; hydrolysis reactions break polymers apart.
C).Dehydration reactions eliminate water from membranes; hydrolosis reactions add water to membranes
D)Hydrolysis reactions create polymers and dehydration reactions create monomers.
B)Dehydration reactions assemble polymers;hydrolosis reactions break polymers apart.
Which of these classes of biological molecules does NOT include polymers?
A)proteins
B)nucleic acids
C)carbohydrates
D)lipids
D)lipids
Which polysaccharide is an important component in the structure of many animals and fungi?
A)cellulose
B)amylopectin
C)chitin
D)anylose
C)chitin
A molecule with the chemical formula C6H12O6 is probably a ____.
A)polysaccharide
B)nucleic acid
C)monosaccharide
D)fatty acid
C)monosaccharide
What is the major structural difference between starch and glycogen?
A)whether glucose is in the α or β form
B)The amount of branching that occurs in the molecule
C)the types of monosaccharide subunits in the molecule
D)they type of glycosidic linkages in the molecule
B)the amount of branching that occurs in the molecule
How do phospholipids interact with water molecules?
A)phospholipids do not interact with water because water is polar and lipids are nonpolar.
B)Phospholipids dissolve in water
C)The polar head interact with water; the nonpolar tails do not
D)the polar heads avoid water; the nonpolar tails attract water (Because water is polar and opposites attract)
C)the polar heads interact with water; the nonpolar tails do not
Lipids ______.
A)are made by dehydration reactions
B)are insoluble in water
C)are made from glycerol, fatty acids, and nitrogen
D)contain less energy than proteins and carbohydrates
B)are insoluble in water

The molecule shown the accompanying figure is a ____.
A)protein
B)steroid
C)phospholipid
D)fatty acid
B)steroid
Saturated fatty acids ______.
A)are usually produced by plants
B)have double bonds between carbon atoms of the fatty acids
C)are usually liquid at room temperature
D)are the principal molecules in lard and butter
D)are the principle molecules in lard and butter
Which one of the following is NOT a component of each monomer used to
make proteins?
A)phosphorus atom, P
B)a side chain, R
C)a carboxyl group, COOH
D)an amino functional group, NH2
A)phosphorus atom P
The tertiary structure of a protein is the ____.
A)unique three-dimensional shape of the fully folded polypeptide
B)overall protein structure resulting from the aggregation of two or more polypeptide subunits
C)order in which amino acids are joined in a polypeptide chain
D)organization of a polypeptide chain into a α-helix or β-pleated sheet
A)unique three-dimensional shape of the fully folded polypeptide
Which level of protein structure do the α-helix or β-pleated sheet represent?
A)primary
B)secondary
C)tertiary
D)quaternary
B)secondary
Which of the following is the strongest evidence that protein structure and function are correlated?
A)proteins function best at certain temperatures
B)denatured (unfolded) proteins do not function normally
C)proteins have four distinct levels of structure and many functions
D)enzymes tend to be globular in shape
B)denature (unfolded) proteins do not function normally
What is the term used for a protein molecule that assists in the proper folding of other proteins?
A)renaturing protein
B)tertiary protein
C)denaturing protein
D)chaperonin
D)chapteronin

The chemical reaction illustrated in the accompanying figure _____.
A)joins 2 fatty acids together
B)is a hydrolysis reaction
C)links 2 polymers to form a monomer
D)results in a peptide bond
D)results in a peptide bond
Which of the following includes all of the pyrimidines found in RNA
and DNA?
A)cytosine and thymine
B)cytosine and uracil
C)cytosine, uracil, and thymine
D)cytosine, uracil, and guanine
C)cytosine, uracil, and thymine
Nucleic acids are polymers made up of which of the following monomers?
A)nucleotides
B)amino acids
C)sugars
D)nitrogenous bases
A)nucleotides
Which of the following descriptions best fits the class of molecules
known as nucleotides?
A)a nitrogenous base and a sugar
B)nitrogenous base and a phosphate group
C)a sugar and a purine or pyrimidine
D)a nitrogenous base, a phosphate group, and a sugar
D)a nitrogenous base, a phosphate group, and a sugar
One of the primary functions of RNA molecules is to _______.
A)function in the synthesis of proteins
B)make a copy of itself, thus ensuring genetic continuity
C)the bases in DNA contain sugars, whereas the bases in RNA do not contain sugars
D)DNA encodes herediary information whereas RNA does not
A)function in the synthesis of proteins
Which of the following statements best summarize the differences between DNA and RNA?
A)DNA contains the base uracil, whereas RNA contains the base thymine
B)DNA nucleotides contain a different sugar than RNA nucleotides
C)The bases in DNA contain sugars, whereas the bases in RNA do not contain sugar
D)DNA encodes heredity information, where as RNA does not
B)DNA nucleotides contain a different sugar than RNA nucleotides
Cell size is limited by _____.
A)the number of proteins within the plasma membrane
B)surface to volume ratios
C)the size of the endomembrane system
D)the surface area of mitochondria in the cytoplasm
B)surface to volume ratios
All of the following are parts of a prokaryotic cell EXCEPT
A)ribosomes
B)an endoplasmic reticulum
C)a plasma membrane
D)a cell well
B)an endoplasmic reticulum
Prokaryotes are classified as belonging to two different domains. hat
are the domains?
A)bacteria and archaea
B)archaea and protista
C)bacteria and protista
D)bacteria and eukarya
A)bacteria and archaea
Which structure is common to plants and animal cells?
A)chloroplast
B)mitochondrion
C)centriole
D)central vacuole
B)mitochondrion
What is the function of the nuclear pore complex found in
eukaryotes?
A)It selectively transports molecules out of the
nucleus, but prevents all inbound molecules from entering the
nucleus
B)It regulates the movement of proteins and RNAs into and
out of the nucleus
C)It assembles ribosomes from raw materials that are synthesized in the nucleus
D)It synthesizes the proteins required to copy DNA and make mRNA
B)It regulates the movement of proteins and RNAs into and out of the nucleus
Which of the following micromolecules leaves the nucleus of a
eukaryotic cell through pores in the nuclear
membrane?
A)DNA
B)Amino acids
C)phospholipids
D)mRNA
D)mRNA
Large numbers of ribosomes are present in cells that specialize in
producing which of the following molecules?
A)glycogen
B)nucleic acids
C)proteins
D)lipids
C)proteins
Which organelle often takes up much of the volume of a plant cell?
A)lysosome
B)Golgi apparatus
C)vacuole
D)peroxisome
C)vacuole
Which structure is NOT part of the endomembrane system?
A)plasma membrane
B)nuclear envelope
C)golgi apparatus
D)cholorplast
D)cholorplast
A cell with an extensive area of smooth endoplasmic reticulum is specialized to _______.
A)synthesize large quantities of lipids
B)import and export protein molecules
C0play a role in storage
D)actively exports protein molecules
A)synthesize large quantities of lipids
Tay-Sachs disease is a human genetic abnormality that results in cells accumulating andbecome clogged with very large, complex, undigested lipids. Which cellular organelle must be involved in this condition?
A)mitochondrion
B)the lysosome
C)Golgi apparatus
D)the endoplasmic reticulus
B)the lysosome
Which structure is the site of the synthesis of proteins that may be exported from the cell?
A)Golgi vesicles
B)roughER
C)free cytoplasmic ribosomes
D)plasmodesmata
B)rough ER
What is the most likely pathway taken by a newly synthesized protein
that will be secreted by a cell?
A)ER -> lysosomes ->
vesicles that fuse with a plasma membrane
B)Golgi -> ER ->lysosome
C)ER -> Golgi -> nucleus
D)ER -> Golgi -> vesiscles that fuse with plasma membranes
D)ER -> Golgi -> vesicles that fuse with plasma membrane
Which organelle is the primary site of ATP synthesis in eukaryotic cells?
A)peroxisome
B)mitochondrion
C)Golgi apparatus
D)lysosome
B)mitochondrion
Cyanide binds with at least one molecule involved in producing ATTP. If a cell is exposed to cyanide, most of the cyanide will be found within the ____.
A)endoplasmic reticulum
B)peroxisomes
C)mitochondria
D)lysosomes
C)mitochondria
In a plant cell, DNA may be found _______.
A)in the nucleus, mitochondria, and chloroplasts
B)only in the nucleus
C)only in the nucleus and chloroplasts
D)in the nucleus, mitochondria, chloroplasts, and peroxisomes
A)in the nucleus, mitochondria, and chloroplasts
Which of the following contain the 9 + 2 arrangement of microtubules,
consisting of 9 doublets of microtubules surrounding a pair of single
microtubles?
A)basal bodies and primary (nonmotile) cilia
B)motile cilia and primary (nonmotile) cilia
C)centrioles and basal bodies
D)flagella and motile cilia
D)flagella and motile cilia
Ions can travel directly from the cytoplasm of one animal cell to the cytoplasm of an adjacent cell through _____.
A)gap junctions
B)desmosomes
C)plasmodesmata
D)tight junctions
A)gap junctions
Which of the following statements about the cytoskeleton is
true?
A)although microtubules are common within a cell, actin
filaments are rarely found outside of the nucleus
B)chemicals that block the assembly of the cytoskeleton would have little effect on a cell's response to external stimuli
C)movement of cilia and flagella is the result of motor proteins causing microtubules to move relative to each other
D)the cytoskeleton of eukaryotes is a static structure most resembling scaffolding used at construction sites
C)movement of cilia and flagella is the result of motor proteins causing microtubules to move relative to each other
Where would you expect to find tight junctions?
A)between plant
cells in a woody plant
B)in the epithelium of an animal's stomach
C)between the smooth endoplasmic reticulum and the rough endoplasmic reticulum
D)in the plasma membrane of prokaryotes
B)in the epithelium of an animal's stomach
Which of the following membrane activities requires energy from
ATP?
A)movement of glucose molecules into a bacterial cell from a
medium containing a higher concentration of glucose than inside the cell
B)movement of carbon dioxide of a paramecium
C)facilitated diffusion of chloride ions across the membrane through a chlorine channel
D)movement of NA+ ions from a lower concentration in a mammalian cell to a higher concentration in the extracellular fluid
D)movement of NA+ ions from a lower concentration in a mammalian cell to a higher concentration in the extracellular fluid
Which term most precisely describes the cellular process of breaking
down large molecules into smaller oes?
A)catabolism (catabolic pathways)
B)anabolism (anabolic pathways)
C)metabolism
D)dehydration
A)catabolism (catabolic pathways)
White blood cells engulf bacteria using _______.
A)receptor mediated endocytosis
B)pinocytosis
C)osmosis
D)phagocytosis
D)phagocytosis
Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as _____.
A)an allosteric activator of the enzyme
B)a noncompetitive inhibitor of the enzyme
C)a coenzyme derived from a vitamin
D)a cofactor necessary for enzyme activity
D)a cofactor necessary for enzyme activity
Which of the following allows water to move much faster across cell membranes?
A)aquaporins
B)ATP
C)peripheral proteins
D)the sodium potassium pump
A)aquaporins
The active site of an enzyme is the region that _______.
A)binds noncompetitive inhibitors of the enzyme
B)is inhibited by the presence of a coenzyme or a cofactor
C)is involved in the catalytic reaction of the enzyme
D)binds allosteric regulators of the enzyme
C)is involved in the catalystic reaction of the enzyme
Which of the following statements is representative of he second law
of thermodynamics?
A)without an input for energy, organisms would
tend toward decreasing entropy
B)cells require a constant input of energy to maintain their high level of organization
C)conversion of energy from one form to another is always accompanied by some gain of free energy.
D)every energy transformation by a cell decreases the entropy of the universe.
B)cells require a constant input of energy to maintain their high level of organization
Which of the following most accurately describes selective permeability?
A)Lipid-soluble molecules pass through a membrane
B)there must be a concentration gradient for molecules to pass through a membrane
C)only certain molecules can cross a membrane
D)an input of energy is required for transport
C)only certain molecules can cross a cell membrane
According to the induced fit hypothesis of enzyme catalysis _____.
A)the binding of the substrate depends on the shape of the active site
B)the active site creates a microenvironment ideal for the reaction
C)some enzymes change their structure when activators bind to the enzyme
D)The binding of the substrate changes the shape of the enzyme's active site.
D)The binding of the substrate changes the shape of the enzyme's active site.
A sodium-potassium pump ______.
A)moves three potassium ions out of a cell and two sodium ions into a cell while consuming 2 ATP in each cycle
B)move three sodium ions out of a cell and two potassium ions into a cell and generate an ATP in each cycle
C)move 3 sodium ions out of a cell and 2 potassium ions into a cell hile consuming ATP for each cycle
D)moves 3 potassium ions out a cell and 2 sodium ions into a cell while producing an ATP for each cycle
C)move 3 sodium ions out of a cell and 2 potassium ions into a cell hile consuming ATP for each cycle
Diffusion ______.
A)is a passive process in which molecules move from a region of higher concentration to a region of lower concentration
B)requires integral proteins in the cell membrane
C)requires an expenditure of energy by the cell
D)is very rapid over long distances
A)is a passive process in which molecules move from a region of higher concentration to a region of lower concentration
The voltage across a membrane is called the ____.
A)electrochemical gradient
B)chemical gradient
C)membrane potential
D)osmotic potential
C)membrane potential
Which of the following would likely move through the lipid bilayer of
a plasma membrane most rapidly?
A)CO2
B)an amino acid
C)glucose
D)K+
A) CO2
Which of the following is true of osmosis?
A)osmosis is an
energy demanding or "active" process.
B)in osmosis, solutes move across a membrane from areas of lower water concentration to areas of higher water concentration
C)osmosis only takes place in red blood cells.
D)in osmosis, water moves across a membrane from areas of lower solute concentration to areas of higher solute concentration.
D)in osmosis, water moves across a membrane from areas of lower solute concentration to areas of higher solute concentration.
A chemical reaction that has a positive ΔG is best described as _____.
A)spontaneous
B)enthalpic
C)endergonic
D)exergonic
C)endergonic
You have isolated a previously unstudied protein, identified its
complete structure in detail, and determined that it catalyzes the
breakdown of a large substrate. You notice it has two binding sites.
One of these is large, apparently the bonding site for the large
substrate; the other is small, possibly a binding site for a
regulatory molecule. What do these findings tell you about the
mechanism of this protein?
A)It is probably an enzyme that works
through competitive inhibition
B)it is probably an enzyme that works through allosteric regulation
C)it is probably a cell membrane transport protein-like an ion channel
D) it is probably a structural protein that is involved in cell-to-cell adhesion
B)it is probably an enzyme that works through allosteric regulation
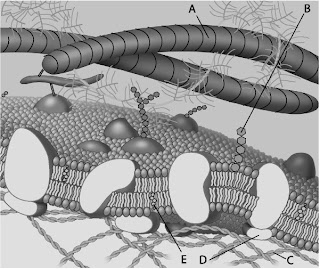
Which component is cholesterol?
A)B
B)C
C)D
D)E
D)E

Which component is a peripheral protein?
A)A
B)B
C)C
D)D
D)D

Which component is a glycolipid?
A)A
B)B
C)C
D)E
B)B
For a protein to be an integral membrane protein, it would have to be ___.
A)hydrophilic
B)amphipathic, with at least one hydrophobic region
C)exposed on only one surface of the membrane
D)hydrophobic
B)amphipathic, with at least one hydrophobic region
Which of these are NOT embedded in the hydrophobic portion of the
lipid bilayer at all?
A)transmembrane proteins
B)integral proteins
C)peripheral proteins
D)all of these are embedded in the hydrophobic portion of the lipid bilayer
C)peripheral proteins

Which curve(s) on the graphs may represent the temperature and pH
profiles of an enzyme taken from a bacterium that lives in a mildly
alkaline hot springs at temperatures of 70°C or higher?
A)
curves 1 and 5
B) curves 2 and 4
C) curves 2 and 5
D) curves 3 and 4
E) curves 3 and 5
A)curves 3 and 5

Which temperature and pH profile curves on the graphs were most likely generated from analysis of an enzyme from a human stomach where conditions are strongly acid.
A) curves 1 and 5
B) curves 1 and 4
C) curves 2 and 4
D) curves 3 and 4
B)curves 1 and 4
A noncompetitive inhibitor decreases the rate of an enzyme reaction by __.
A)changing the shape of the enzyme's active site
B)acting as a coenzyme for the reaction
C)binding at the active site for the enzyme
D)changing the free energy change of the reaction.
A)changing the shape of the enzyme's active site
HIV is the virus that causes AIDS. In the mid-1990s, researchers
discovered an enzyme in HIV called protease. Once the
enzyme's structure was known, researchers began looking for drugs that
would fit into the active site and block it. If this strategy for
stopping HIV infections were successful, it would be an example of
what phenomenon?
A)denaturation
B)vaccination
C)competitive inhibition
D)allosteric regulation
C)competitive inhibition
What kind of molecules pass through a cell membrane most
easily?
A)small and hydrophobic
B)large and hydrophobic
C)large polar
D)ionic
A)small and hydrophobic
The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is most precisely described as _____.
A)allosteric inhibition
B)feedback inhibition
C)metabolic inhibition
D)noncooperative inhibition
B)feedback inhibition
You have discovered an enzyme that can catalyze two different
chemical reactions. Which of the following is most likely to be
correct?
A)the enzyme is subject to competitive inhibition and
allosteric regulation.
B)Two types of allosteric regulation occur: the binding of one molecule activates the enzyme, while the binding of a different molecule inhibits it.
C)the enzyme contains alpha-helixes and beta-pleated sheets.
D)Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in shape and size.
D)Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in shape and size.
The lock and key model analogy for enzymes applies to the specificity of enzymes _____.
A)interacting with ions
B)binding to their substrate
C)as they form their tertiary and quaternary structure
D)interacting with water
B)binding to their substrate
Which of the following is true of enzymes?
A)enzymes increase the rate of chemical reaction by providing activation energy to the substrate.
B)enzymes increase the rate of chemical reaction by providing activation energy to the substrate.
C)enzymes increase the rate of chemical reaction by lowering activation energy barriers
D)enzyme function is increased if the 3D structure or conformation of an enzyme is altered.
C)enzymes increase the rate of chemical reaction by lowering activation energy barriers
Anabolic pathways ____.
A)release energy as they degrade from polymers to monomers
B)consume energy to decrease the entropy of the organism and its environment
C)are usually highly spontaneous chemical reactions
D)consume energy to build up polymers from monomers
D)consume energy to build up polymers and monomers
Which of the following processes includes all the others?
A)osmosis
B)facilitated diffusion
C)transport of an ion down its electrochemical gradient
D)passive transport
D)passive transport
Which of the following is true for all exergonic reactions
A)the reaction proceeds with a net release of free energy
B)the reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.
C)a net input of energy from the surroundings is required for the reactions to proceed.
D) the products have more total energy than the reactants.
A)the reaction proceeds with a net release of free energy
A patient was involved in a serious accident and lost a large quantity of blood. In an attempt to replenish body fluids, distilled water--equal to the volume of blood lost--is added to the blood directly via one of his veins. What will be the most probable result of this transfusion?
A)the patients red blood cells will burst because the blood has become hypertonic compared to the cells.
B)the patients red blood cells will shrivel up because the blood has become hypertonic compared to the cells
C)the patients red blood cells will shrivel up because the boood has become hypotonic compared to the cells
D)the patients red blood cells will swell and possibly burst because the blood has become hypotonic compared to the cells.
D)the patients red blood cells will swell and possibly burst because the blood has become hypotonic compared to the cells.
The force driving simple diffusion is _____, while the energy source for active transport is _____.
A)phosphorylated protein carriers; ATP
B)transmembrane pumps; electron transport
C)the concentration gradient; ATP
D)the concentration gradient; ADP
C)the concentration gradient; ATP
Which of the following is a characteristic feature of a carrier protein in a plasma membrane?
A)it works against diffusion
B)it requires the expenditure of cellular energy to function
C)it exhibits a specificity for a particular type of molecule
D)it has no hydrophobic regions
C)it exhibits a specificity for a particular type of molecule

How do cells use the ATP cycle shown in the figure?
A)cells use the cycle to recycle ADP, phosphate, and the energy by ATP hydrolysis.
B)cells use the cycle to recycle energy released by ATP hydroylsis
C)cells use the cycle to recycle ADP and phosphate
D)cells use the cycle primarily to generate heat
C)cells use the cycle to recycle ADP and phosphate
Which of the following is the most correct interpretation of the figure?
A)ATP is a molecule that acts as an intermediary to store energy for cellular work,
B)Pi acts as a shuttle molecule to move energy from ATP to ADP
C) Energy from catabolism can be used directly for performing cellular work.
D)ATP + Pi are set of molecules that store energy for catabolism
A)ATP is a molecule that acts as an intermediary to store energy for cellular work,
Which of the following is a statement of the first law of thermodynamics?
A)energy cannot be transferred or transformed
B)energy cannot be created or destroyed
C)the entropy of the universe is decreasing
D)the entropy of the universe is constant
B)energy cannot be created or destroyed
The difference between pinocytosis and receptor-mediated endocytosis is that _____.
A)pinocytosis is nonselective in the molecules it brings into the cell, whereas receptor mediated endocytosis offers more selectivity
B)pinocytosis increases the surface area of the plasma membrane, where as receptor mediated endocytosis decreases the plasma membrane surface area
C)pinocytosis brings only water molecules into the cell, but receptor mediated endocytosis brings in other molecules as well
D)pinocytosis can concentrate substances from the extra cellular fluid, but receptor mediated endocytosis cannot
A)pinocytosis is nonselective in the molecules it brings into the cell, whereas receptor mediated endocytosis offers more selectivity.
When a glucose molecule loses a hydrogen atom as the result of an oxidation-reduction reaction, the molecule becomes _____.
A)reduced
B)hydrolyzed
C)oxidized
D)an oxidizing agent
C)oxidized
When a molecule of NAD+ gains a hydrogen atom (not a proton) the molecule becomes _____.
A)dehydrogenated
B)reduced
C)oxidized
D)redoxed
B)reduced
The oxygen consumed during cellular respiration is involved directly in which process or event?
A)gycolosis
B)accepting electrons at the end of the electron transport chain
C)the oxidation of pyruvate to acetyl CoA
D)the citric acid cycle
B)accepting electrons st the end of the electron transport chain
Where does the process of glycolysis occur in the cell?
A)cytoplasm
B)mitochondria matrix
C)inner mitochondrial membrane
D)christae
A)cytoplasm
Starting with one molecule of glucose, the energy-containing products of glycolysis are _____.
A)2 NADH, 2 pyruvate, and 2 ATP
B) 6CO2, 2 pyruvate, and 2 ATP
C)2 FADH2, 2 pyruvate, and 4 ATP
D)2 NAD+, 2 pyruvate, and 2 ATP
A)2 NADH, 2 pyruvate, and 2 ATP
In glycolysis, for each molecule of glucose oxidized to pyruvate ______.
A)two molecules of ATP are used and four molecules of ATP are produced
B)two molecules of ATP are used and six muldcules of ATP are produced
C)two molecules of ATP are used and two molecules of ATP are produced
D)four molecules of ATP are used and two molecules of ATP are produced
A)two molecules of ATP are used and four molecules of ATP are produced
Pyruvate must be converted into what molecule for it to enter the citric acid cycle?
A)oxaloacetate
B)G3P
C)acetyl co-A
D)ferradoxin
C)acetyl co-A
Which electron carriers function in the cytric acid cycle?
A)NADH and FADH2
B)NAD+ only
C)ADP and ATP
D)the electron transport chain
A)NADH and FADH2
For each turn of the citric acid cycle, how many of each molecule are produced?
A)1 ATP, 3 NADH, 1 FADH2
B)0 ATP, 3 NADH, 1 FADH2
C)2 ATP, 6 NADH, 2 FADH2
D)2 ATP, 1 NADH, 3 FADH2
A)1 ATP, 3 NADH, 1 FADH2
Where in the cell does the citric acid cycle take place?
A)mitochondrial matrix
B)cytopalsm
C)inner mitochondrial membrane
D)nucleus
A)mitochondrial matrix
Where are the proteins of the electron transport chain located?
A)mitochondrial outer membrane
B)mitochondrial intermembrane space
C)mitochondrial matrix
D)mitochondrial inner membrane
D)mitochondrial inner membrane
Which of the following events takes place in the electron transport chain?
A)the breakdown of glucose into two pyruvate molecules
B)substrate-level phosphorlyation
C)the extraction of energy from high-energy electrons remaining from glycolysis and the citric acid cycld
D)the breakdown of an acetyl group to carbon dioxide
C)the extraction of energy from high energy electrons remaining from glycolysis and the citric acid cycle
During aerobic respiration, electrons travel downhill in which sequence
A)glucose -> ATP -> electron transport chain -> NADH
B)glucose -> NADH -> electron transport chain -> oxygen
C)food -> glycolysis ->citric acid cycle -> NADH -> ATP
D)glucose -> pyruvate -> ATP -> oxygen
B)glucose -> NADH -> electron transport chain -> oxygen
Approximately how many molecule of ATP are produced from the complete oxidation of one molecule of glucose (C6H12O6) in aerobic cellular respiration?
A)2
B)30-32
C)4
D)18-24
B)30-32
Which of the following normally occurs of whether or not oxygen (O 2) is present?
A)oxidative phosphorylation (chemiosmosis)
B)citric acid cycle
C)fermentation
D)glycolysis
D)glycolysis
Which of the following occurs in the cytosol of a eukaryotic cell?
A)oxidation of pyruvate to acytol CoA
B)citric acid cycle
C)glycolysis and fermentation
D)fermentation and chemiosmosis
C)glycolysis and fermentation
In the absence of oxygen, yeast cells can obtain energy by fermentation, resulting in the production of
A)ATP, CO2, ethanol (ethyl alcohol)
B)ATP, NADH, and pyruvate
C)ATP, CO2, and lactate
D)ATP, pyruvate, and acetyl CoA
A)ATP, CO2, ethanol (ethyl alcohol)
Why is glycolysis considered to be one of the first metabolic pathways to have evolved?
A)it does not involve organelles or specialized structured, does not require oxygen, and is present in most organisms
B)it requires the presence of membrane- enclosed organelles are found only in eukaryotic celk
C)it produces much less ATP than does oxidative phosphorylation
D)it is found in prokaryotic cells but not in eukaryotic cells
A)it does not involve organelles or specialized structured, does not require oxygen, and is present in most organisms
The process of photosynthesis probably originated ____.
A)in plants
B)three separate times duringe evolution
C)in prokaryotes
D)in fungi
C)in prokaryotes
Plants in photosynthesize _____.
A)and respire only in the light
B)only in the light but respire in light and dark
C)only in the dark but respire only in the light
D)only in the light but respire only in the dark
B)only in the light but respire in light and dark
Early investigators thought the oxygen produced by photosynthesis plants came from carbon ____.
A)air
B)glucose
C)electrons from NADPH
D)water
D) water

The figure shows the absorption spectrum for chlorophyll a and the action spectrum for photosynthesis. Why are they different?
A)Other pigments absorb light in addition to chlorophyll a.
B)Green and yellow wavelengths inhibit the absorption of red and blue wavelengths.
C)Oxygen given off during photosynthesis interfere with the absorption of light
D)aerobic bacteria take up oxygen, which changes the measurement of the rate of photosynthesis
A)other pigments absorb light in addition to chlorophyll a

What wavelength of light in the figure is most effective in driving photosynthesis?
A)625mm
B)420mm
C)575mm
D)730mm
B)420mm
A spaceship is designed to support animals life for a multiyear voyage to the outer planets of the solar system. Plants will be grown to provide oxygen and to recycle carbon dioxide since the spaceship will be too far from the sun for photosynthesis, an artificial light source will be needed.
A)green light
B)a mixture of blue and red light
C)UV light
D)full spectrum white light
B)a mixture of blue and red light
Suppose a plant has a unique photosynthesis pigment and the leaves of this plant appear to be reddish yellow. What wavelengths of visible light are absorbed by this pigment?
A)red and yellow
B)green and yellow
C)blue and violet
D)blue, green, and yellow
C)blue and violet
What event accompanies energy absorption by chlorophyll (or other pigment molecules of the antenna complex)?
A)ATP is synthesized of energy absorbed
B)A carboxylation reaction of the Calvin cycle occurs.
C)an electron is excited.
D)electrons are stripped from NADPH
C)an electron is excited.
What are the products of linear electron flow?
A)ATP and P700
B)heat and fluorescense
C)ATP and NADPH
D)ADP and NADPH
C)ATP and NADPH
What are the products of cyclic electron flow?
A)ATP and P700
B)ATP and NADPH
C)ATP only
D)ADP and NADP+
C)ATP only
In a plant cell, where are the ATP synthase complexes located?
A)inner mitochondrial membrane only
B)thylakoid membrane and plasma membrane
C)thylakoid membrane only
D)thylakoid membrane and inner mitochondrial membrane
D)thylakoid membrane and inner mitochondrial membrane
In mitochondria, chemiosmosis moves protons from the matrix into the intermembrane space, whereas in chloroplasts, chemiosmosis moves protons from the _____.
A)thylakoid space to the stroma
B)matrix to the stroma
C)intermembrane space in the matrix
D)stroma to the thylakoid space
D)stroma to the thylakoid space
Which of the following statements best describes the relationship between photosynthesis and respiration?
A)photosynthesis occurs only in plants; respiration occurs only in animals
B)photosynthesis stores energy in complex organic molecules; respiration releases energy from complex organic molecukes
C)photosynthesis in catabolic; respiration in anabolic
D)respiration runs the biochemical pathways of photosynthesis in reverse
B)photosynthesis stores energy in complex organic molecules; respiration releases from complex organic molecules
In a plant, the reactions that produce molecular oxygen (O2) take place in ____.
A)the light reactions alone
B)neither the light reactions nor the Calvin cycle
C)the light reactions and the Calvin cycle
D)the Calvin cycle alone
A)the light reactions alone
Which of the following are products of the light reactions of photosynthesis that are utilized in the Calvin cycle?
A)ADP, Pi, and NADP+
B)ATP and NADPH
C)CO2 and glucose
D)H2O and O2
B)ATP and NADPH
Where does the Calvin cycle take place?
A)interior of the thylakoid (thylakoid space)
B)thylakoids membrsne
C)stroma of the chloroplast
D)outer membrane of the chloroplast
C)stroma of the chloroplast
What is the primary function of the Calvin cycle?
A)synthesize simple sugars from carbon dioxide
B) split water and release oxygen
C) transport RuBP out of the chloroplast
D)use NADPH to release carbon dioxide
A)synthesize simple sugars from carbon dioxide
The alternative pathways of photosynthesis using the C4 or CAM systems are said to be compromises. Why?
A)CAM plants allow more water loss, while C4 plants allow less CO2 into the plant.
B)C4 compromises on water loss and CAM compromises on photorespiration
C)Each one minimizes both water loss and rate of photosynthesis
D)Both minimize photorespiration but expend more ATP during carbon fixation
D)Both minimize photorespiration but expend more ATP during carbon fixation
Photorespiration _____.
A) consumes carbon dioxide and generate ATP, sugars, and oxygen
B)generates carbon dioxide and consumes ATP and oxygen
C)generates ATP and sugars and consumes oxygen and carbon dioxide
D)generates oxygen and consumes ATP, carbon dioxide, and sugars
B)generates carbon dioxide and consumes ATP and oxygen
Every ecosystem must have ____.
A)producers and primary consumers
B)photosynthesizers
C) autotrophs
D)autotrophs and heterotrophs
C)autotrophs
Which of the following does NOT occur during the Calvin cycle?
A)regeneration of the CO2 acceptor
B)consumption of ATP
C)release of oxygen
D)oxidation of NADPH
C)release of oxygen
CAM plants keep stomata closed in the daytime, thus reducing loss of water. They can do this because they ____.
A)fix CO2 into sugars in the bundle-sheath cells
B)fix CO2 into pyruvate in the mesophyll cells
C)fix CO2 into organic acids during the night
D)use photosystem I and photosystem II at night
C)fix CO2 into organic acids during the night
In yeast signal transduction, a yeast cell released a mating factor which ______.
A)passes through the membranes of neighboring cells, bind to DNA, and initiates transcription
B)diffuses through the membranes of distant cells, causing them to produce factors that initiate long distance migrations
C)acts back on the same cell that secreted the mating factor, changing its development
D)binds to receptors on the membranes of other types of yeast cells
D)binds to receptors on the membranes of other types of yeast cells
Which of the following is a type of a local signaling in which a cell secretes a signal molecule that affects neighboring cells?
A)a synaptic signaling
B)paraffins signaling
C)autocrine sigjaling
D)hormonal signaling
b)paracrine signaling
Hormones are chemical substances produced in one organ that are released into the bloodstream and affect the function of a target organ. For the target organ to respond to a particular hormone, it must ____.
A)have receptors that recognize and bind the hormone molecule
B)be from the same cell type as the organ that produces the hormone
C)modify its plasma membrane to alter the hormone entering the cytoplasm
D)experience an imbalance that disrupts its normal function
A)have receptors that recognize and bind the hormone molecule
A G-protein receptor with a GTP bound to it ____.
A)Directly affects gene expression
B)signals a protein to maintain its shape and conformation
C)will use cGMP as a second messenger
D)is in its active state
D)is in its active state
One of the major categories of receptors in the plasma membrane reacts by forming diners, adding phosphate group, and then activating relay proteins. Which type does this?
A)steroid receptors
B)G protein-coupled receptors
C)ligand-gated ion channels
D)receptor tyrosine kinases
D)receptor tyrosine kinases
Binding of a signaling molecule to which type of receptor leads directly to a change in the distribution of ions on opposite sides of the membrane?
A)ligand-gated ion channel
B)receptor tyrosine kinase
C)intracelluoar receptor
D)g protein coupled receptor
A)ligand-gated ion channel
If an animal cell suddenly lost the ability to produce GTP, what might happen to its signaling system?
A)it would employ a transduction pathway directly from an external messenger
B)it would use ATP instead of GTP to activate and inactivate the G protein on the cytoplasmic side of the plasma membrane
C)It would not be able to activate and inactivate G protein on the cytoplasmic side of the plasma membrane
D)it would be able to carry out reception and transduction but would not be able to respond t is signal.
C)It would not be able to activate and inactivate G protein on the cytoplasmic side of the plasma membrane
Which of the following would be inhibited by a drug that specifically blocks the addition of phosphate groups to proteins?
A)G-protein coupled receptor bindinf
B)adenylyl cyclase activity
C)receptor tyrosine kinase activity
D)ligand gated ion channel signaling
C)receptor tyrosine kinase activity
What does it mean to say that a signal is transducer?
A)the signal enters the cell directly and binds to a receptor inside
B)the signal is amplified, such than ever a signal molecule evokes a large response
C)the signal triggers a sequence of phosphorylation events inside the cell
D)the physical form of the signal changes from one form to another
D)the physical form of the signal changes from one form to another
In general, a signal transmitted via phosphorlyation of a series of proteins _____.
A)results in a conformational change to each protein
B)activates a transcription event
C)requires binding of a hormone to a intracellular receptor
D)generates ATP in the process of signal transduction
A)activates a transcription event
Protein kinases is an enzyme that ____.
A)activates or inactivated other proteins by adding a phosphate group to them
B)functions as a second messenger molecules
C)produces second messenger molecules
D)generates ATP in the process of signal transduction
A)activates or inactivated other proteins by adding a phosphate group to them
Protein kinase is an enzyme that ____.
A)activates or inactivated other proteins by adding a phosphate group to them
B)functions as a second messenger molecule
C)produces second messenger molecules
D)serves as a receptor for various signal molecules
A)activates or inactivated other proteins by adding a phosphate group to them
In signal transduction, phosphatases ___.
A)inactivated protein kinases and turn off the signal transduction
B)prevent a protein kinase from being reused when there is another extracellular signal
C)move the phosphate group of the transduction pathway
D)amplify the second messengers such as cAMP
A)inactivate protein kinases and turn off the signal transduction
Put the steps of the process of signal transduction in the order they occur:
1. A conformational change in the signal receptor complex activates an enzyme
2 protein kinases are activated
3. A signal molecule mines to a receptor
4. Target proteins are phosphorylated
5. Second messenger molecules are released
A)3,1, 2, 4, 5
B)1, 2, 5, 3, 4
C)3, 1, 5, 2, 4
D)1, 2, 3, 4, 5
C)3, 1, 5, 2, 4
Transcription factors _____.
A)control gene expression
B)regulate the synthesis of DNA in respeomde to a signal
C)transcribe ATP into cAMP
D)regulate the synthesis of lipids in the cytoplasm
A)control gene expression
Scaffolding proteins are ____.
A)microtubular proteins arrays that allow lipid-soluble hormones to get from the cell membrane to the nuclear pores
B)large molecules to which several relay proteins attach to facilitate cascade effects
C)proteins that can reach into the nucleus of a cell to affect transcription
D)relay proteins that orient receptors and their ligand in appropriate directions to facilitate their complexing.
B)large molecules to which several relay proteins attach to facilitate cascade effects
Phosphorylation cascades involving a series of protein kinases are useful for cellular signal transduction because they _____.
A)counter the harmful effects of phosphatases
B)are species specific
C)amplify the original signal many times
D)always lead to the same cellular response
C)amplify the original signal many times
Which of the following describes the events of apoptosis?
A)the cells DNA and organelles become fragmented, the cell shrinks and forms blebs, and the cells parts are packaged in vesicles are digested by specialized cells
B)the cells nucleus and organelles are lysed, then the cell enlarged and burst
C)the cell dies, it is lysed, its organelles are phagocytozed, and its contents are recycled
D)the cells DNA and organelles become fragmented, the cell dies, and its phagocytized
A)the cells DNA and organelles become fragmented, the cell shrinks and forms blebs, and the cells parts are packaged in vesicles are digested by specialized cells
Apoptosis involves all butt which of the following?
A)digestion of cellular contents by scavenger cells
B)fragmentation of the DNA
C)lysis of the cell
D)activation of cellular enzymes
C)lysis of the cell
Cells that are infected, damaged, or have reached the end of their functional life span often undergo programmed cell death. This controlled cell suicide is called apoptosis. Select the appropriate description of this event on a cells life cycle
A)apoptosis is regulated by cell surface receptors that signal when a cell has reached its density-dependent limits
B)during apoptosis, cellular agents chop up DNA and fragment the organelles and other cytoplasmic components of a cell
C)during apoptosis, dying cells leak out their contents including digestive enzymes that also destroy healthy cells
D)each cell organelles has protein signals that initiate the breakdown of the organelles components which lead to cell death
B)during apoptosis, cellular agents chop up DNA and fragment the organelles and other cytoplasmic components of a cell

Which of the following types of signaling is represented in the figure
A)synaptic
B)autocrine
C)hormonal
D)paracrine
A)synaptic

In the figure, the dots in the space between the two strictures represent which of the following?
A)hormomes
B)neurotransmitters
C)receptor molecules
D)signal transducers
B)neurotransmitters
Which of the following is true of steroid receptors?
A)the receptor molecuoes are themselves lipids or glycolipids
B)steroid receptors are typically bound to the external surface of the nuclear envelope
C)the receptor may be inside the nucleus of a target cell
D)the unbound steroid receptors are quickly recycled by lysosomes
C)the receptor may be inside the nucleus of a target cell
What is the name of the signaling molecule that binds to a receptor to cause a respone?
A)integrin
B)transductor
C)ligand
D)hormone
C)ligand
Which of the following is NOT an example of a second messenger?
A)PEP
B)IP3
C)DAG
D)Ca+2
A)PEP
When a signal molecule leaves a receptor, the receptor does what?
A)response is activated
B)signal is amplified
C)response is terminated
D)signal is transduced
C)response is terminated