Distillation is the separation of multiple _____ components based on their different ______.
As the mixture is heated and the first component ____, its _____ form travels through the distillation set-up and ______ into a different container.
liquid, boiling points.
boils, vapor, condenses.
To insert a thermometer into an adapter, use _____ to prepare the thermometer. Then, hold the thermometer _____ the adapter and _____ the thermometer into the adapter.
mineral oil, close to, slowly turn
What are the concerns presented by overheating a distillation to a dry flask?
-> The empty glassware might heat quickly, igniting vapors from the distillation.
-> The remaining solid residue might contain explosive peroxides.
Before turning on the heat for a microscale distillation, what should you confirm about the set-up?
-> Secure connections at the joints
-> Presence of boiling chip in the sample
-> Existence of some opening in the set-up
Where should the tip of the thermometer be placed in a microscale distillation set-up?
At or slightly below the side arm of the distillation head
An azeotrope is a mixture that has _____ composition in the __________. Therefore, an azeotropic mixture _____ be separated by distillation.
the same, gas and liquid phases.
cannot
Suppose you are using distillation to separate cyclohexane and toulene. The boiling point of cyclohexane is ____ oC and the boiling point of toulene is ____ oC. Therefore, the liquid collected first should be ________.
81, 111, cyclohexane
Lab#2
Most desired compound from one layer to another ____
Often involves a reaction in one of the layers _____
Leaves desired compound in its starting layer _____
Movies impurities from one layer to another ______
Leaves impurities in their starting layer _____
1. Extraction
2. Extraction
3. Wash
4. Wash
5. Extraction
Suppose you are performing an extraction procedure with a carboxylic acid, like benzoic or toluic acid.
Which layer should contain the carboxylic acid in a two-layer mixture of water and an organic solvent, like diethyl ether?
The organic layer
You then add a base to form the corresponding carboxylate.
Which layer should contain the carboxylate in a two-layer mixture of water and organic solvent, like diethyl ether?
The aqueous layer
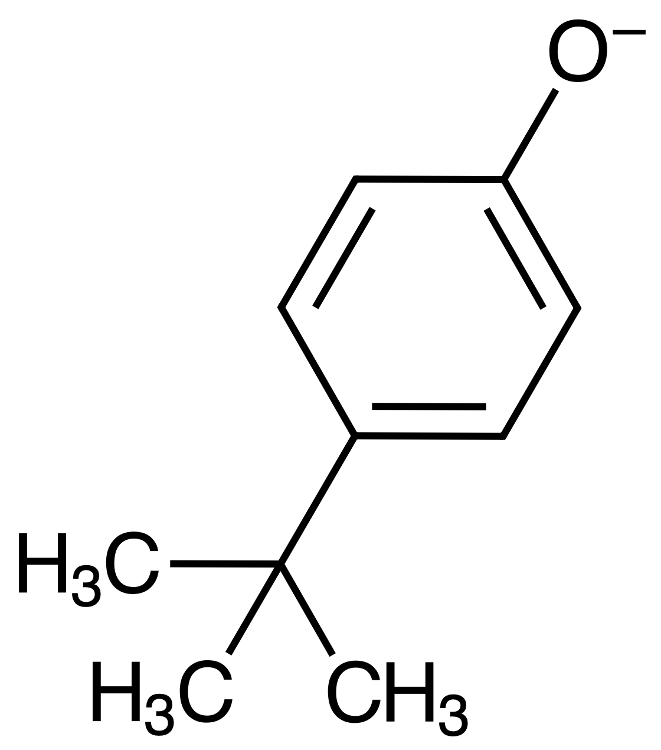
Suppose you are performing an acid/base extraction. After one of the steps, you have collected a NaOH extract containing phenolate.
Next, you add HCl to the layer. What are the products of the HCl solution?
The acid can donate a proton to the phenolate, forming phenol. Chloride ion is left after the acid loses a proton.
PhO- + HCl ----> PhOH + Cl-
What is the purpose of a drying agent in the work up of an organic reaction?
To absorb small amounts of water in an organic solution
When using acids and bases, note that these substances are ______. Make every effort to avoid contact with ___________. Be sure to wipe up any spills ________.
corrosive, the skin and lab surfaces, immediately
Aniline involves an amine, which is a _____ functional group. When an aqueous acid solution is added to an organic solution including aniline, the aniline appears in the ______ layer in its ______ form. Then, a base is added to ________ the aniline.
basic, aqueous, protonated, reconstitute
Identify the solution used for each described purpose.
Reconstitute aniline from aqueous layer ____
Reconstitute benzoic acid out out of the aqueous layer ______
Extract benzoic acid into the aqueous layer ____
Extract reconstituted 3-nitroaniline out of the aqueous layer ____
Extract aniline into the aqueous layer _____
1. 6 M NaOH
2. 6 M HCl
3. Dichloromethane
4. 5% NaOH solution
5. Dichloromethane
6. 5% HCl solution
What hazards are associated with napthalene?
-> Naphthalene is flammable
-> Naphthalene produces nauseating vapors
-> Naphthalene is toxic
Lab #3
Before adding a sample or solvent to a separatory funnel, what should you have in place?
-> A stopcock in the closed position
-> A funnel in the top of the separatory funnel
-> A flask under the separatory funnel
After mixing solutions in a separatory funnel, the stopper should be _____ and the liquid should be ________ and the layers allowed to separate.
When you get close to the interface between the layers, _______ the funnel and _______ until the first layer is collected. _______ to collect the second layer.
removed, drained through the stopcock
get eye level with, slow the draining
switch to a new flask
After an organic reaction involving an aqueous solution, the organic solution might be washed with a saturated sodium chloride aqueous solution, known as brine.
What is the purpose of the brine wash?
To reduce the amount of water in the organic solution.
What is the visual indicator that enough of a drying agent, such as anhydrous MgSO4 or CaCl2, has been added to properly dry an organic solution?
The drying agent will move freely like a powder around the solution.
After drying an organic solution, what methods can be used to remove the drying agent from the solution?
-> Decanting
-> Vacuum or gravity filtration
If crystal growth does not start on its own after the solution in the flask returns to room temperature, identify the best ways to promote this process.
-> Add a bit of solid as a seed crystal.
-> Scratch the bottom of the flask gently with a stirring rod.
What is the best course of action if solid material remains in the flask after the heating step of a recrystallization?
Filter the hot mixture.
In general, when hydrocarbons like oil are added to water, the two liquids ______ because hydrocarbons are _____ and water is ______.
do not mix, non-polar, polar.
Identify the characteristics of a good recrystallization solvent.
-> Dissolves a sample well at high temperatures
-> Does not dissolve a sample well at low temperatures
Identify the best support for a separatory funnel.
Iron ring clamped to a ring stand.
When using a separatory funnel, you should burp the funnel.
What does the therm burping mean?
Opening the stopcock when the flask is inverted during mixing.
When drying an organic solution, start by transferring the solution to ________ or a test tube, depending on the amount.
Then, add ________ of the drying agent and ______ to determine if you added enough drying agent.
Continue _______ until the solution is dry.
an Erlenmeyer flask
pea-sized amount, swirl the flask
adding small amounts
Identify the best drying agent or process for each described purpose.
Removal of small amounts of water from a polar solvent _______
Removal of visible pockets of water from an organic solvent ______
Storage of solvents or other materials in a desiccator _____
Anhydrous calcium chloride
Brine wash
Drierite
Lab#4
After a recrystallization, a pure substance will ideally appear as a network of _______. If this is not the case, it may be worthwhile to reheat the flask and allow the contents to cool more ______.
large crystals, slowly
In order to dissolve a chemical sample in a recrystallization solvent, add the room- temperature solvent _________ in a Erlenmeyer flask on a hot plate. Turn on the heat, starting at ___________. Using a _____, add additional solvent from a second container on the heat source. Swirl the sample flask after each addition, and try to add __________ in order to dissolve the solid.
just until it covers the sample
a low setting and increasing gradually
pipet
as little solvent as possible
What property is the basis of the purification technique recrystallization?
Solubility
After a mixture has been poured through filter paper, the ______ component remains on top while the _____ flows through. To collect the solid, spread the filer paper _______ and allow the solvent to ______.
solid, liquid, on a watch glass, evaporate.
After assembling a gravity filtration apparatus, begin the separation of the mixture by _____ it into the filter paper. Pour the entire contents of the mixture into the filter paper, ______ the solid in the filter paper if needed to speed up filtration. After all the liquid has drained through the filter paper, finish by rinsing the solid with ______.
decanting, stirring, solvent.
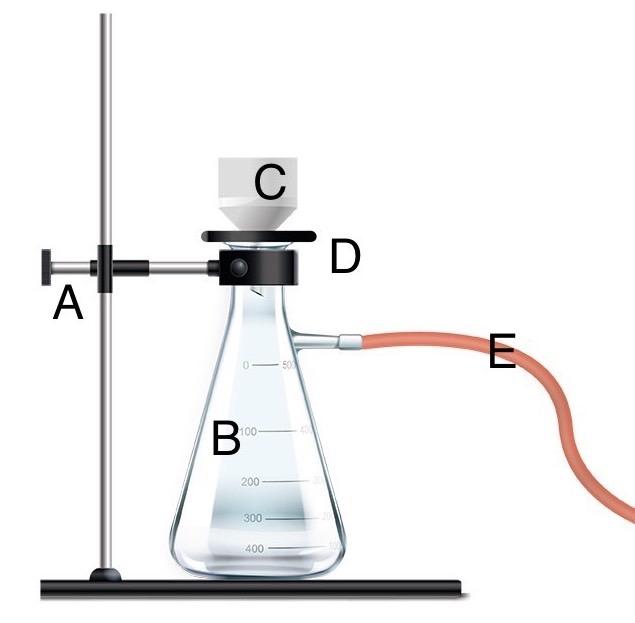
Using letters on the imagine, identify each component of the vacuum filtration set up.
A - Clamp
B - Side-arm flask
C - Buchner funnel
D - Adapter
E - Vacuum tubing
You can separate a mixture of sand and copper(II) sulfate pentahydrate using filtraton. What difference in properties enables the separation of the two solids by filtration?
The hydrate is soluble in water but the sand is not.
Sulfuric acid, H2SO4, is an important industrial chemical, typically synthesized in a multi-step process. What is the percent yield if a batch of H2SO4 has a theoretical yield of 3.6 kg, and 2.7 kg are obtained at the end of the process?
75%
percent yield = actual yield / theoretical yield x 100%
Why might activated carbon be added to a sample during the recrystallization process?
To absorb impurities causing the recrystallization solution to have an incorrect color.
When using vacuum filtration to separate a dissolved solid from an undissolved solid, what techniques should you use to ensure a a quantitative separation?
-> Dry the solid on the filter paper after the separation
-> Wet the filter paper before pouring the mixture into the filter funnel.
Lab#5
At the melting point, the solid form of a substance _________ the liquid form of the substance.
is in equilibrium with
1. Experimental melting point is close to literature value____
2. Experimental melting point is below literature value_____
3. Wide melting point range_____
4. Narrow melting point range______
1 & 4 : Pure sample of a single compound
2 & 3 : Impure sample of multiple compounds.
To prepare a sample in a capillary tube for a melting point determination, gently tap the tube into the sample with _____ end of the tube down. Continue tapping until the sample is ________ . Then, with the _____ end of the tube down, tap the sample down slowly or ____________ to move the sample down faster.
Finally, make sure that you can see _________ in the magnifier when placed in the melting point apparatus before turning on the heat.
open
couple of millimeters high
closed, drop the tube into a longer tube
some or all of the solid
Why should you use a finely ground solid when determining the melting point of a sample?
-> Air pockets in a coarse sample could disrupt heat distribution.
-> Uniform, small particles heat more consistently throughout the sample.
What characteristics should a good sample for melting point determination have?
-> Solid phase
-> Thoroughly dry
-> Small particles
What is the recommended order of measurements to report the most accurate melting point possible?
Use quick heating to estimate the melting point _____
Use slow heating to carefully observe melting _____
Use slow heating to confirm the careful measurement ____
First step
Second step
Third step
When performing a melting point determination, how should you report your findings?
As a rang from the temperature when melting starts to the temperature when it ends.
When melting mixtures of compounds, what is the eutectic composition?
The mixture composition with the lowest melting point.