An element is defined as a substance that:
cannot be broken into simpler substances by chemical reactions.
Which of the following elements is NOT responsible for a significant portion of the mass of living organisms?
S.
An atom has six protons and eight neutrons. Its atomic mass is __ atomic mass units.
fourteen.
The difference between a stable isotope and a radioisotope is that:
the radioisotope emits radiation.
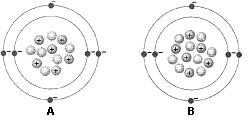
Figure 2-1 Use the figure below to answer the corresponding question(s). The atomic mass of the atom identified as A in Figure 2-1 is:
12.
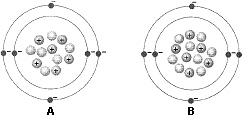
Figure 2-1 Use the figure below to answer the corresponding question(s). Figure 2-1 represents:
two isotopes of the same element.

Figure 2-1 Use the figure below to answer the corresponding question(s). The difference between the two atoms in Figure 2-1 is:
the number of neutrons.
Isotopes differ from each other with respect to the number of:
neutrons only.
Radioisotopes are used in all of the following scientific applications except:
measuring the pH of the blood.
The chemical behavior of an atom is determined by the:
number of valence electrons.
Which of the following statements is FALSE?
The 2nd energy level contains a maximum of 10 electrons.
Chlorine has seven electrons in its valence shell. The number of electrons it must gain to complete its valence shell is:
1.
Any chemical interaction between atoms:
involves only valence electrons.
The representation H-O-H is known as:
a structural formula.
The molecular mass of C6H12O6 is 180 amu. 0.25 moles of this substance contain:
45 g.
How many molecules are present in one mole of C6H12O6?
6.02 x 1023 molecules.
In a chemical reaction, the product is:
generally written on the right side and is the substance generated by the reaction.
When a chemical reaction is at dynamic equilibrium:
the forward and reverse reactions are proceeding at equal rates.
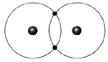
Figure 2-2 Use the figure below to answer the corresponding question(s). Figure 2-2 represents:
molecular hydrogen.
Figure 2-2 Use the figure below to answer the corresponding question(s). The type of bond illustrated in Figure 2-2 is:
a single covalent bond.
Which covalent bond involves only 2 electrons:
single.
A covalent bond:
may be polar or nonpolar depending on the atoms involved.
In a water molecule, because oxygen is more electronegative than hydrogen, the shared electrons are more commonly found around the __ nucleus more often than the __ nucleus.
oxygen; hydrogen.
The covalent bond between a hydrogen atom and the oxygen atom in water is formed when:
hydrogen and oxygen share an electron pair.
Covalently bonded atoms with similar electronegativities are:
nonpolar.
An atom becomes a cation if:
it loses one or more electron.
The difference between an electrically neutral atom and an ion is that:
an ion has an unequal number of protons and electrons, while an atom has an equal number.
In the formation of common table salt, sodium and chlorine interact because:
sodium donates one electron to chlorine.
Table salt dissolves easily in water because:
the partial positive charge of the hydrogens in the water molecule can associate with the negative charge of the chloride ion, and the partial negative charge of the oxygen of the water molecule can associate with the positive charge of the sodium atom.
The process whereby water molecules surround ions during the process of dissolving is called:
hydration.
Which of the following statements concerning van der Waals interactions is FALSE:
they are very strong.
Which component becomes oxidized in the following chemical reaction? 4 Fe + 3 O2 → 2 Fe2O3
iron.
Which component is the oxidizing agent in the following chemical reaction? 4 Fe + 3 O2 → 2 Fe2O3
oxygen.
The cohesiveness between water molecules is due largely to:
hydrogen bonds.
A stalk of celery is placed in a solution of blue colored dye. After one hour, the leaves have blue fluid in their veins. Which property of water is being demonstrated?
adhesion and cohesion.

Which characteristic of water molecules directly contributes to the remarkable “water walking" success of the aquatic insects pictured in the accompanying figure?
hydrogen bonds.
Which of the following statements is not correct?
Water heats up and cools down very quickly.
It takes 1 calorie of heat to raise the temperature of 1 gram of water 1 degree Celsius at sea level. This is referred to as the __ of water.
specific heat.
Evaporative cooling is a process whereby __ moving __ molecules vaporize, thus __ large amounts of heat.
fast; water; removing.
At what temperature is water most dense?
4 degrees Celsius.
This characteristic of a molecule determines the ability of hydrogen bonds to form between it and hydrogen:
an atom with a partial negative charge.
Which characteristic of water makes the existence of pH possible?
ionization.
A pH of 4 is __ times more __ than a pH of 7.
1000; acidic.
What is the OH- concentration of a solution having a pH of 2?
1 x 10-12.
When a small amount of hydrochloric acid (HCl) is added to a solution of Na2HPO4, the pH of the solution does not change markedly. The pH also does not change drastically when a small amount of sodium hydroxide (NaOH) is added to this same solution. Based on these observations, the compound Na2HPO4 is:
acting as a buffer.
A salt is a compound in which the hydrogen ion of __ is replaced by some other cation.
an acid.
A solution having a pH of 6 would:
be slightly acidic.
Identify the chemical(s) that act(s) as a buffer in human blood:
bicarbonate.
Identify the hydrogen ion concentration that represents the lowest pH from the following list:
1 x 10-3.
Which of the following has a pH closest to that of human blood?
sea water.