Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Campbell Biology Chapter 05 (powell_h)
front 1 Humans and mice differ because | back 1 Answer: D |
front 2 Molecules with which functional groups may form polymers via
dehydration reactions? | back 2 Answer: E |
front 3 Which of these molecules is not formed by dehydration reactions?
| back 3 Answer: A |
front 4 In animal metabolism, most of the monomers released by digestion of
food macromolecules are metabolized to provide energy. Only a small
portion of these monomers are used for synthesis of new
macromolecules. The net result is that | back 4 Answer: B |
front 5 Which of these classes of biological molecules consist of both small
molecules and macromolecular polymers? | back 5 Answer: B |
front 6 Which of the following is not a polymer? | back 6 Answer: A |
front 7 What is the chemical reaction mechanism by which cells make polymers
from monomers? | back 7 Answer: C |
front 8 How many molecules of water are needed to completely hydrolyze a
polymer that is 11 monomers long? | back 8 Answer: C |
front 9 Which of the following best summarizes the relationship between
dehydration reactions and hydrolysis? | back 9 Answer: A |
front 10 Which of the following polymers contain nitrogen? | back 10 Answer: D |
front 11 The molecular formula for glucose is C₆H₁2O₆. What would be the
molecular formula for a molecule made by linking three glucose
molecules together by dehydration reactions? | back 11 Answer: B |
front 12 The enzyme amylase can break glycosidic linkages between glucose
monomers only if the monomers are the α form. Which of the following
could amylase break down? | back 12 Answer: A |
front 13 On food packages, to what does the term insoluble fiber refer?
| back 13 Answer: A |
front 14 A molecule with the chemical formula C₆H₁₂O₆ is probably a | back 14 Answer: E |
front 15 Lactose, a sugar in milk, is composed of one glucose molecule joined
by a glycosidic linkage to one galactose molecule. How is lactose
classified? | back 15 Answer: D |
front 16 All of the following are polysaccharides except | back 16 Answer: A |
front 17 Which of the following is true of both starch and cellulose? | back 17 Answer: A |
front 18 Which of the following is true of cellulose? | back 18 Answer: D |
front 19 Humans can digest starch but not cellulose because | back 19 Answer: C |
front 20 Which of the following statements concerning saturated fats is not
true? | back 20 Answer: B |
front 21 A molecule with the formula C₁₈H3₆O₂ is probably a | back 21 Answer: B |
front 22 Which of the following statements is true for the class of biological
molecules known as lipids? | back 22 Answer: A |
front 23 The label on a container of margarine lists "hydrogenated
vegetable oil" as the major ingredient. What is the result of
adding hydrogens to vegetable oil? | back 23 Answer: B |
front 24 Which of the following is true regarding saturated fatty acids?
| back 24 Answer: C |
front 25 Large organic molecules are usually assembled by polymerization of a
few kinds of simple subunits. Which of the following is an exception
to this statement? | back 25 Answer: A |
front 26 Which modifications of fatty acids will best keep triglycerides solid
at warmer temperatures? | back 26 Answer: D |
front 27 Why are human sex hormones considered to be lipids? | back 27 Answer: B |
front 28 All of the following contain amino acids except | back 28 Answer: B |
front 29 The bonding of two amino acid molecules to form a larger molecule
requires | back 29 Answer: A |
front 30 There are 20 different amino acids. What makes one amino acid
different from another? | back 30 Answer: C |
front 31 The bonding of two amino acid molecules to form a larger molecule
requires which of the following? | back 31 Answer: A |
front 32 Polysaccharides, triacylglycerides, and proteins are similar in that
they | back 32 Answer: B |
front 33 Dehydration reactions are used in forming which of the following
compounds? | back 33 Answer: E |
front 34 Upon chemical analysis, a particular polypeptide was found to contain
100 amino acids. How many peptide bonds are present in this protein?
| back 34 Answer: C |
front 35 What aspects of protein structure are stabilized or assisted by
hydrogen bonds? | back 35 Answer: E |
front 36 How many different kinds of polypeptides, each composed of 12 amino
acids, could be synthesized using the 20 common amino acids? | back 36 Answer: E |
front 37 Which bonds are created during the formation of the primary structure
of a protein? | back 37 Answer: A |
front 38 What maintains the secondary structure of a protein? | back 38 Answer: B |
front 39 Which type of interaction stabilizes the α helix and the β pleated
sheet structures of proteins? | back 39 Answer: D |
front 40 Which level of protein structure do the α helix and the β pleated
sheet represent? | back 40 Answer: B |
front 41 The amino acids of the protein keratin are arranged predominantly in
an α helix. This secondary structure is stabilized by | back 41 Answer: E |
front 42 The tertiary structure of a protein is the | back 42 Answer: C |
front 43 What type of covalent bond between amino acid side chains (R groups)
functions in maintaining a polypeptide's specific three-dimensional
shape? | back 43 Answer: D |
front 44 At which level of protein structure are interactions between the side
chains (R groups) most important? | back 44 Answer: C |
front 45 The R group or side chain of the amino acid serine is –CH₂–OH. The R
group or side chain of the amino acid leucine is –CH₂–CH–(CH₃)₂. Where
would you expect to find these amino acids in a globular protein in
aqueous solution? | back 45 Answer: B |
front 46 Misfolding of polypeptides is a serious problem in cells. Which of
the following diseases are associated with an accumulation of
misfolded polypeptides? | back 46 Answer: D |
front 47 Changing a single amino acid in a protein consisting of 325 amino
acids would | back 47 Answer: E |
front 48 Normal hemoglobin is a tetramer, consisting of two molecules of β
hemoglobin and two molecules of α hemoglobin. In sickle-cell disease,
as a result of a single amino acid change, the mutant hemoglobin
tetramers associate with each other and assemble into large fibers.
Based on this information alone, we can conclude that sickle-cell
hemoglobin exhibits | back 48 Answer: E |
front 49 What methods may be used to elucidate the structures of purified
proteins? | back 49 Answer: E |
front 50 In a normal cellular protein, where would you expect to find a
hydrophobic amino acid like valine? | back 50 Answer: D |
front 51 Which of the following techniques uses the amino acid sequences of
polypeptides to predict a protein's three-dimensional structure?
| back 51 Answer: B |
front 52 If cells are grown in a medium containing radioactive ³⁵S, which of
these molecules will be labeled? | back 52 Answer: C |
front 53 What is the term used for a protein molecule that assists in the
proper folding of other proteins? | back 53 Answer: B |
front 54 DNAase is an enzyme that catalyzes the hydrolysis of the covalent
bonds that join nucleotides together. What would first happen to DNA
molecules treated with DNAase? | back 54 Answer: B |
front 55 Which of the following statements about the 5' end of a
polynucleotide strand of DNA is correct? | back 55 Answer: B |
front 56 One of the primary functions of RNA molecules is to | back 56 Answer: B |
front 57 If ¹⁴C-labeled uridine triphosphate is added to the growth medium of
cells, what macromolecules will be labeled? | back 57 Answer: C |
front 58 Which of the following descriptions best fits the class of molecules
known as nucleotides? | back 58 Answer: C |
front 59 Which of the following are nitrogenous bases of the pyrimidine type?
| back 59 Answer: B |
front 60 Which of the following are nitrogenous bases of the purine type?
| back 60 Answer: B |
front 61 If a DNA sample were composed of 10% thymine, what would be the
percentage of guanine? | back 61 Answer: C |
front 62 A double-stranded DNA molecule contains a total of 120 purines and
120 pyrimidines. This DNA molecule could be composed of | back 62 Answer: B |
front 63 The difference between the sugar in DNA and the sugar in RNA is that
the sugar in DNA | back 63 Answer: E |
front 64 Which of the following statements best summarizes the differences
between DNA and RNA? | back 64 Answer: C |
front 65 If one strand of a DNA molecule has the sequence of bases 5'ATTGCA3',
the other complementary strand would have the sequence | back 65 Answer: B |
front 66 What is the structural feature that allows DNA to replicate? | back 66 Answer: B |
front 67 A new organism is discovered in the forests of Costa Rica. Scientists
there determine that the polypeptide sequence of hemoglobin from the
new organism has 72 amino acid differences from humans, 65 differences
from a gibbon, 49 differences from a rat, and 5 differences from a
frog. These data suggest that the new organism | back 67 Answer: B |
front 68 Which of the following is an example of hydrolysis? | back 68 Answer: D |
front 69 If cells are grown in a medium containing radioactive ³²P-labeled
phosphate, which of these molecules will be labeled? | back 69 Answer: E |
front 70 If cells are grown in a medium containing radioactive ¹⁵N, which of
these molecules will be labeled? | back 70 Answer: E |
front 71 How will brief heating (to 95°C) affect macromolecular structures in
aqueous solution? | back 71 Answer: E |
front 72 Which of the following is not a monomer/polymer pairing? | back 72 Answer: C |
front 73  If two molecules of the general type shown in Figure 5.1 were linked
together, carbon-1 of one molecule to carbon-4 of the other, the
single molecule that would result would be | back 73 Answer: A |
front 74  Which of the following descriptors is true of the molecule shown in
Figure 5.1? | back 74 Answer: E |
front 75  Which of the following statements is true regarding the molecule
illustrated in Figure 5.2? | back 75 Answer: D |
front 76  Which of the following statements is true regarding the molecule
illustrated in Figure 5.3? | back 76 Answer: C |
front 77 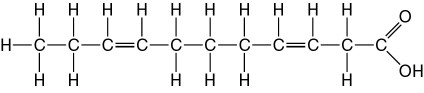 The molecule shown in Figure 5.3 is a | back 77 Answer: E |
front 78  What is the structure shown in Figure 5.4? | back 78 Answer: C |
front 79  Which of the following statements is/are true regarding the chemical
reaction illustrated in Figure 5.5? | back 79 Answer: B |
front 80  At which bond would water need to be added to achieve hydrolysis of
the peptide, back to its component amino acid? | back 80 Answer: C |
front 81  Which bond is a peptide bond? | back 81 Answer: C |
front 82  Which bond is closest to the amino terminus of the molecule? | back 82 Answer: A |
front 83 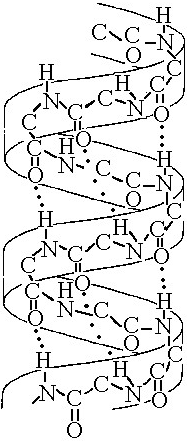 The structure depicted in Figure 5.7 shows the | back 83 Answer: D |
front 84  Which molecule has both hydrophilic and hydrophobic properties and
would be found in plasma membranes? | back 84 Answer: B |
front 85  Which of the following combinations could be linked together to form
a nucleotide? | back 85 Answer: D |
front 86  Which of the following molecules contain(s) an aldehyde type of
carbonyl functional group? | back 86 Answer: E |
front 87  Which molecule is glycerol? | back 87 Answer: C |
front 88  Which molecule is a saturated fatty acid? | back 88 Answer: E |
front 89  Which of the following molecules is a purine type of nitrogenous
base? | back 89 Answer: E |
front 90  Which of the following molecules act as building blocks (monomers) of
polypeptides? | back 90 Answer: B |
front 91  Which of the following molecules is an amino acid with a hydrophobic
R group or side chain? | back 91 Answer: B |
front 92  Which of the following molecules could be joined together by a
peptide bond as a result of a dehydration reaction? | back 92 Answer: C |
front 93  A fat (or triacylglycerol) would be formed as a result of a
dehydration reaction between | back 93 Answer: B |
front 94  Which of the following molecules could be joined together by a
phosphodiester type of covalent bond? | back 94 Answer: D |
front 95  Which of the following molecules is the pentose sugar found in RNA?
| back 95 Answer: D |
front 96  Which of the following molecules contains a glycosidic linkage type
of covalent bond? | back 96 Answer: E |
front 97  Which of the following molecules has a functional group that
frequently forms covalent bonds that maintain the tertiary structure
of a protein? | back 97 Answer: A |
front 98  Which of the following molecules consists of a hydrophilic
"head" region and a hydrophobic "tail" region?
| back 98 Answer: B |
front 99  Which of the following statements is false? | back 99 Answer: E |
front 100 Approximately 32 different monomeric carbohydrate subunits are found
in various natural polysaccharides. Proteins are composed of 20
different amino acids. DNA and RNA are each synthesized from four
nucleotides. | back 100 Answer: C |
front 101 Which class of biological polymer has the greatest functional
variety? | back 101 Answer: B |
front 102 Professor Jamey Marth at the University of California, Santa Barbara,
identified 70 molecules that are used to build cellular macromolecules
and structures. These include at least 34 saccharides, 8 nucleosides,
and 20 amino acids. In theory, then, which class of biological polymer
has the greatest information-coding capacity? | back 102 Answer: A |
front 103 Which of the following categories includes all others in the list?
| back 103 Answer: D |
front 104 The enzyme amylase can break glycosidic linkages between glucose
monomers only if the monomers are in the α form. Which of the
following could amylase break down? | back 104 Answer: A |
front 105 Which of the following statements concerning unsaturated fats is
true? | back 105 Answer: B |
front 106 The structural level of a protein least affected by a disruption in
hydrogen bonding is the | back 106 Answer: A |
front 107 Enzymes that break down DNA catalyze the hydrolysis of the covalent
bonds that join nucleotides together. What would happen to DNA
molecules treated with these enzymes? | back 107 Answer: B |
front 108 The molecular formula for glucose is C₆H₁₂O₆. What would be the
molecular formula for a polymer made by linking ten glucose molecules
together by dehydration reactions? | back 108 Answer: C |
front 109 Which of the following pairs of base sequences could form a short
stretch of a normal double helix of DNA? | back 109 Answer: D |