Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Colorimetric Determination of an Equilibrium Constant in an Aqueous Solution
front 1 Purpose of this experiment | back 1 become familiar w/ the concept of equilibrium by determination of an equilibrium constant for a reaction in a solution |
front 2 Characteristic of all chemical equilibria | back 2 dynamic and constantly proceeding in both directions |
front 3 State of equilibrium | back 3 when the rate of the reaction in the forward direction is equal to the rate of the reaction in the reverse direction forward rate of reaction = reverse rate of reaction |
front 4 What will we use as a source of Fe3+ ions? SCN- ions? | back 4 iron (III) nitrate, Fe(NO3)3 sodium thiocyanate, NaSCN |
front 5 Why will we use 0.10M nitric acid instead of water? | back 5 Because equilibrium constants are dependent on the total ionic concentration of the equilibrium mixture. By using nitric acid we ensure that all mixtures have comparable ionic concentrations. equilibrium constants are also temperature dependent, so we'll record the temp at which our measurements are made |
front 6 What is the reaction for the formation of FeNCS2? | back 6  Fe3+(aq) + SCN-(aq) <--> FeSCN2+ Pale yellow colorless blood red *Note: this is the simplified version of the equation. The Fe3+ is in reality [Fe(H2O)6]3+ and FeNCS2+ is actually [Fe(SCN)(H2O)5]2+ |
front 7 Equilibrium constant expression for the formation of FeNCS2+? | back 7  Keq= [FeSCN2+]/ [Fe3+][SCN-] |
front 8 Example of the calculation for equilibrium constant (Keq) expression for the formation of FeNCS2+. | back 8  |
front 9 Example of ICE table used for FeNCS2+ | back 9  |
front 10 What are the five components of a spectrophotometer? | back 10 1. light source producing light w/ a wavelength from about 375-650 nm 2. a monochromator- selects a particular wavelength of light and sends it to the sample cell w/ an intensity of I0 3. the sample cell- contains the solution being analyzed 4. a detector- measures the intensity, I, of the light transmitted from the sample cell 5. a meter- indicates the intensity of the transmitted light |
front 11 For a given substance, the amount of light absorbed depends on: (4) | back 11 1. concentration 2. cell or path length 3. wavelength of light 4. solvent |
front 12 Absorption spectra | back 12  plots of the amount of light absorbed versus wavelength |
front 13 Two ways of expressing the amount of light absorbed? | back 13 1. Percent transmittance, %T 2. Absorbance, A |
front 14 Expression for percent transmittance, %T | back 14  %T corresponds to the percentage of light transmitted When the sample in the cell is a solution, I= intensity of light transmitted by the solution; I0= intensity of light transmitted when the cell only contains solvent |
front 15 Expression for absorbance, A? | back 15 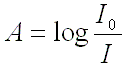 If there is no absorption of light at any given wavelength, the %T is 100 and the absorbance is 0. On the other hand, if the sample absorbs all of the light, the %T is 0 and the absorption is ∞ (infinity) |
front 16 Term synonymous w/ absorbance | back 16 optical density, OD |
front 17 How is absorbance related to concentration | back 17 by the Beer-Lambert Law: A= abc A= absorbance b= solution path length c= concentration (mol/L) a= molar absorptivity or molar coefficient |
front 18 What is the relationship between absorbance and concentration when the Beer-Law is obeyed? | back 18 there is a linear relationship between absorbance and concentration However, since deviations from this law occasionally occur, it is wise to construct a calibration curve of absorbance vs. concentration |