Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
STUDY GUIDE chapter 1,2,3
front 1 Phase of metabolism that provides energy by BREAKING DOWN COMPLEX MOLECULES into SIMPLE MOLECULES. (e.g.) PROTEINS → AMINO ACIDS | back 1 CATABOLISM |
front 2 ISOTOPES | back 2
|
front 3 Heterogeneous, will settle | back 3 Suspensions |
front 4 What type of homeostatic feedback reflex is the withdrawal reflex? | back 4 negative |
front 5 An atom with ________ electrons could be an anion when ionically bonded. | back 5 ANSWER: 9 |
front 6 in certain kinds of muscle cells, calcium ions are stored | back 6 in the smooth ER |
front 7 An example of a coenzyme is | back 7 riboflavin (vitamin B2) |
front 8 What is a DIPOLE? | back 8 POLAR MOLECULE |
front 9 Two good examples of a colloid would be Jell-O® or | back 9 cytosol |
front 10 Phase of metabolism that uses the energy from CATABOLISM to build up the BODY'S STRUCTURAL and FUNCTIONAL COMPONENTS. It is also called BIOSYNTHESIS. (e.g.) AMINO ACIDS → PROTEINS. | back 10 ANABOLISM |
front 11 The coiling of the protein chain backbone into an alpha helix is referred to as the ________. | back 11 secondary structure |
front 12 Which of the following are subdivisions of anatomy? | back 12  regional, systemic, and surface |
front 13 A solution that has a pH of 2 could best be described as being ________. | back 13 acidic |
front 14 In a DNA molecule, the phosphate serves | back 14 to hold the molecular backbone together |
front 15 The numbers listed represent the first, second, and third energy levels, respectively. On this basis, which of the following is an unstable or reactive atom? | back 15 2, 8, 1 |
front 16 The ATP molecule is not used in? | back 16
chemical work |
front 17 Which of the following is a neutralization reaction? | back 17 HCl + NaOH → NaCl + H2O |
front 18 What is the pathway between the receptor and the control center in the reflex pathway called? | back 18 afferent pathway |
front 19  In certain kinds of muscle cells, calcium ions are stored in | back 19 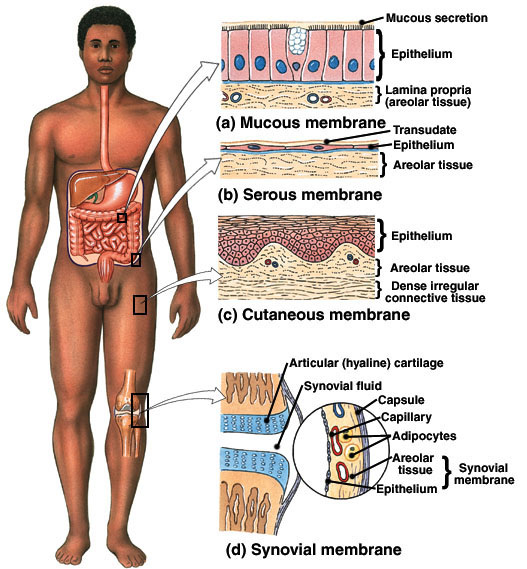 the smooth ER |
front 20
| back 20
|
front 21 If an atom were to have two protons, then it would | back 21 be very stable |
front 22 Which body system would be most affected by a lower than normal atmospheric pressure? | back 22 respiratory system |
front 23 Most fibrous proteins in the body contain all of these except: | back 23 keratin |
front 24  Can lungs carry out excretory functions? Explain your answer. | back 24  Yes, carbon dioxide is a metabolic waste the lungs excrete. |
front 25
| back 25
|
front 26 HYDROGEN | back 26 Which bonds often bind different parts of a molecule into a specific three-dimensional shape? |
front 27
In liquid XYZ, you notice that light is scattered as it
passes through. There is no precipitant in Heterogeneous, will not settle | back 27 colloid |
front 28 
| back 28 
|
front 29  Which of the following is not considered a factor in influencing a reaction rate? | back 29  TIME |
front 30 In redox reactions ________. | back 30 both decomposition and electron exchange occur |
front 31 What is a vertical section through the body, dividing it into anterior and posterior regions called? | back 31 FRONTAL |
front 32
Select which reactions will usually be irreversible
regarding chemical equilibrium in living | back 32 glucose to CO2 & H2O |
front 33 Select the statement about mixtures that is correct. | back 33
A) A
solution contains solvent in large amounts and solute in
smaller quantities. Answer: A |
front 34  What broad term covers all chemical reactions that occur within the body cells? | back 34  metabolism |
front 35 Fibrous proteins | back 35 are very stable and insoluble in water |
front 36 Which of the following is true regarding the concentration of solutions? | back 36
A) Percent solutions are parts per 1000 parts. Answer: B |
front 37 
| back 37 
|
front 38 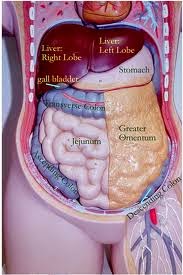
| back 38 
|
front 39 
| back 39 
|
front 40 
| back 40 
|
front 41 The single most abundant protein in the body is ________. | back 41 COLLAGEN |
front 42 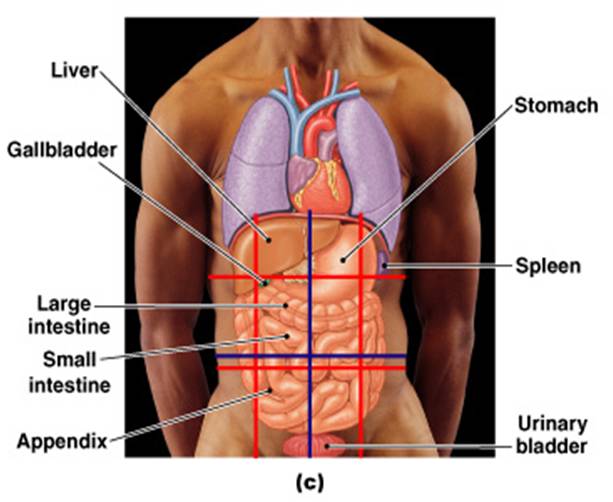
| back 42 
|
front 43 .gif) THE FOLLOWING DESCRIBE ENZYMES? | back 43 .gif)
|
front 44 The parietal pleura would represent a serous membrane | back 44 LINING THE THORACIC CAVITY |
front 45 Smallest particle of an element that retains its properties. | back 45 ATOM |
front 46 If cells are placed in a hypertonic solution containing a solute to which the membrane is impermeable, what could happen? | back 46 The cells will lose water and shrink. |
front 47 What moves cell organelles from one location to another inside a cell? | back 47 Motor proteins |
front 48 The basic structural material of the body consists of | back 48 PROTEINS |
front 49  Help prevent molecules from passing through the extracellular space between adjacent cells. | back 49  Tight junctions |
front 50 An amino acid may act as a proton acceptor or donor. | back 50 Amino acids have two components--a base group (proton acceptor) and an organic acid part (a proton donor). Some have additional base or acid groups on the ends of their R groups as well. |
front 51 A dipeptide can be broken into two amino acids by dehydration synthesis. | back 51 TRUE |
front 52 In general, the lipids that we refer to as oils have ________. | back 52 a high degree of unsaturated bonds |
front 53 Many plasma proteins may function as _________. | back 53 BUFFERS |
front 54 Hydrogen bonds are too weak to bind atoms together to form molecules but are important intramolecular bonds | back 54 TRUE |
front 55 Which one of the following systems responds fastest to environmental stimuli? | back 55 NERVOUS |
front 56
| back 56 Transfer RNA Messenger RNA |
front 57 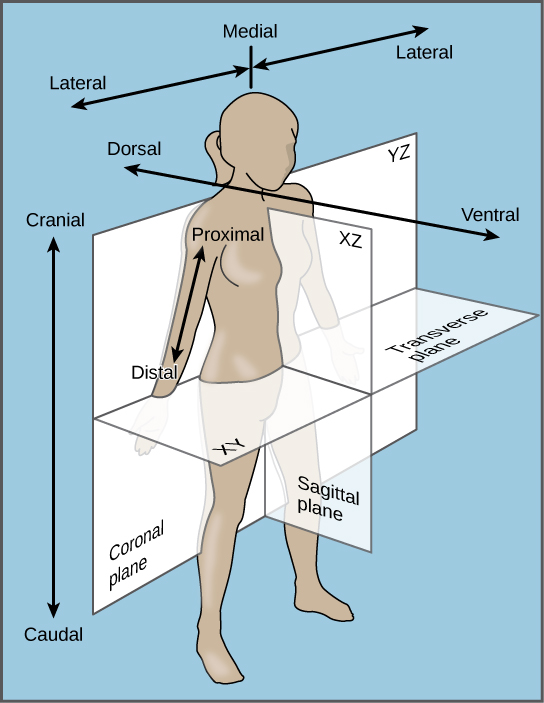
| back 57 
|
front 58 Which structures are fingerlike projections that greatly increase the absorbing surface of cells? | back 58 MICROVILLI |
front 59 May be attached to the ER or scattered in the cytoplasm | back 59 Synthetase enzymes |
front 60
| back 60
|
front 61 
| back 61 
|
front 62 Anything that occupies space and has mass. | back 62 MATTER |
front 63 Although a man who weighs 175 pounds on Earth would be lighter on the moon and heavier on Jupiter, his ________ would not be different. | back 63 MASS |
front 64 Which of the following is not true of proteins? | back 64 They appear to be the molecular carriers of coded hereditary information. |
front 65 Coenzymes are ________. | back 65 organic molecules derived from vitamins |
front 66
| back 66
|
front 67 What type of chemical bond can form between an element with 11 protons and an element with 17 protons? | back 67 IONIC |
front 68 Carbohydrates and proteins are built up from their basic building blocks by the ________. | back 68 removal of a water molecule between each two units |
front 69
| back 69
|
front 70 Is a function of, and varies with, gravity. | back 70 WEIGHT |
front 71 Dry ice (frozen carbon dioxide). | back 71 COMPOUND |
front 72 Which of the following describes the plasma membrane? | back 72 a phospholipid bilayer surrounding the cell |
front 73 Legs moving the pedals of a bicycle. | back 73 MECHANICAL |
front 74 When the bonds of ATP are broken, energy is released to do cellular work. | back 74 Chemical energy |
front 75  Can be measured only by its effects on matter | back 75  ENERGY |
front 76 The lower the pH, the higher the hydrogen ion concentration. | back 76 TRUE |
front 77 Are able to detoxify substances by enzymatic action. | back 77 Peroxisomes |
front 78 Are hollow tubes made of spherical protein subunits called tubulins. Answer: | back 78 Microtubules |
front 79 Carbon | back 79 element |
front 80 Which of the following is not a role of molecular chaperonins? | back 80 act as a platform for assembling primary protein structure |
front 81 Represented by the flow of charged particles along a conductor, or the flow of ions across a membrane. | back 81 Electrical energy |
front 82 Which of the following does NOT describe enzymes? | back 82 Enzymes work by raising the energy of activation. |
front 83  What does the polar end of a phospholipid contain? | back 83  phosphorus-containing group |
front 84 Which metals have a toxic effect on the body? | back 84 heavy metals such as bismuth, Most often the definition of toxic metals includes at least cadmium, manganese, lead, mercury and the radioactive metals. |
front 85 Removes and filters excess fluid from tissues. | back 85 Lymphatic |
front 86
| back 86
|
front 87 
| back 87 
|
front 88
| back 88
|
front 89 Which feedback mechanism causes the variable to deviate further and further from its original value or range? Answer: | back 89 POSTIVE FEEDBACK |
front 90
| back 90
|
front 91
| back 91
|
front 92 
| back 92
|
front 93
| back 93
|
front 94 
| back 94 
|
front 95 The higher we go in the mountains, the greater the atmospheric pressure, resulting in an increase in available oxygen. Comment on this statement. | back 95 At high altitudes, the atmospheric pressure is less than at lower levels resulting in a decrease in oxygen levels. The lower oxygen levels may be inadequate to support cellular metabolism. |
front 96 Which of the following is NOT a function of the smooth endoplasmic reticulum? | back 96 protein synthesis in conjunction with ribosomes |
front 97 A holoenzyme is composed of an apoenzyme and a(n) ________. Answer: | back 97 cofactor |
front 98
| back 98
|
front 99  Why are the abdominopelvic cavity organs the most vulnerable to blunt deceleration in an automobile accident with seat belts? | back 99  The walls of the abdominal cavity are formed only by trunk muscles and are not reinforced by bone. The pelvic organs receive a somewhat greater degree of protection from the bony pelvis. |
front 100 What can happen when the usual negative feedback mechanisms are overwhelmed and destructive positive feedback mechanisms take over? | back 100 Homeostatic imbalances increase our risk for illness and produce the changes we associate with aging |
front 101 Which of the following is not a factor that binds cells together? | back 101 glycolipids in the glycocalyx |
front 102 Hydrogen bonds are more like a type of weak ________ than true bonds. | back 102 attraction |
front 103
| back 103
|
front 104 Current information suggests that omega-3 fatty acids decrease the risk of heart disease. | back 104 TRUE |
front 105 Which of these is an inclusion, not an organelle? | back 105
cilia |
front 106 Combined number of protons and neutrons in an atom | back 106 Mass number of an element |
front 107
| back 107
|
front 108 Electrically charged particle due to loss of an electron. | back 108 CATION |
front 109
| back 109
|
front 110
| back 110
|
front 111
| back 111
|
front 112
| back 112
|
front 113
| back 113
|
front 114
| back 114
|
front 115
| back 115
|
front 116
| back 116
|
front 117  Which of the following statements is most correct regarding the intracellular chemical signals known as "second messengers"? | back 117  Cyclic AMP and calcium may be second messengers. |
front 118 
| back 118 
|
front 119
| back 119
|
front 120
| back 120
|
front 121  Crenation (shrinking) is likely to occur in blood cells immersed in ______. Caveolae are closely associated with all of the following? | back 121 
|
front 122
| back 122
|
front 123
| back 123
|
front 124 Describe two important functions of the Golgi apparatus. | back 124 To modify, sort, and package proteins. |
front 125
| back 125
|
front 126
| back 126
|
front 127
| back 127
|
front 128 Explain the term genetic code. What does it code for? What are the letters of the code? | back 128 The genetic code is the information encoded in the nucleotide base sequence of DNA. A sequence of three bases, called a triplet, specifies an amino acid in a protein. The letters of the code are the four nucleotide bases of DNA designated as A, T, C, and G. |
front 129 
| back 129 
|
front 130
| back 130
|
front 131
| back 131
|
front 132
| back 132
|
front 133 What is the common route of entry for flu viruses into a cell? | back 133 Flu viruses and diphtheria toxins use receptor-mediated endocytosis. The virus can attach to the receptors or to the substances the receptors accept to "hitch a ride" into the cell. |
front 134 How are peroxisomes different from lysosomes? | back 134 Peroxisomes contain oxidases that use oxygen to detoxify harmful substances. They are very good at neutralizing free radicals. Peroxisomes directly bud from the ER. Lysosomes contain powerful hydrolytic enzymes that will pretty much destroy anything they come in contact with. They are manufactured by the Golgi apparatus. |
front 135 Briefly name the subphases of interphase and tell what they do. | back 135 G1 - growth phase. The cell is metabolically active and the centriole begins to divide at the end of this phase. S - DNA replicates itself. New histones are made and assembled into chromatin. G2 - Enzymes and proteins are synthesized and centriole replication is completed. This is the final phase of interphase. |
front 136
| back 136
|
front 137
| back 137
|
front 138
| back 138
|
front 139
| back 139
|
front 140
| back 140
|
front 141
| back 141
|
front 142 
| back 142 
|
front 143
| back 143
|
front 144
| back 144
|
front 145  The ability to sense changes in the environment and respond to them is called?. | back 145  responsiveness or excitability |
front 146 .gif) What is the ratio of fatty acids to glycerol in neutral fats? Neutral fats have a________ ratio of fatty acids to glycerol. | back 146 .gif) 3:1 |
front 147 is fat soluble, produced in the skin on exposure to UV radiation, and necessary for normal bone growth and function | back 147 VITAMIN D |
front 148 The four elements that make up about 96% of body matter are | back 148 carbon, oxygen, hydrogen, nitrogen |
front 149 Molecules such as methane that are made of atoms that share electrons have ________ bonds. Answer: | back 149 covalent |
front 150  Atom X has 17 protons. How many electrons are in its valence shell? | back 150  7 |
front 151 Which property of water is demonstrated when we sweat? | back 151 high heat of vaporization |
front 152 Which protein types are vitally important to cell function in all types of stressful circumstances? | back 152 molecular chaperones |
front 153 If atom X has an atomic number of 74 it would have which of the following? | back 153 74 protons |
front 154 The atomic weight is only an average of relative weights of an atom and its isotopes, and it may vary from the weight of a specific isotope | back 154 TRUE |
front 155 What does the formula C6H12O6 mean | back 155 There are, 6 carbon, 12 hydrogen, and 6 oxygen atoms. |
front 156 Explain the difference between potential and kinetic energy. | back 156 Potential energy is inactive stored energy that has potential to do work. Kinetic energy is energy in action. |
front 157 How can phospholipids form a film when mixed in water? | back 157 Phospholipids have both polar and nonpolar ends. The polar end interacts with water, leaving the nonpolar end oriented in the opposite direction. |
front 158 Select the most correct statement regarding nucleic acids. | back 158 DNA is a long, double-stranded molecule made up of A, T, G, and C bases. |
front 159 Which of the following statements is false? | back 159 A)The more hydrogen ions in a solution, the more acidic the solution. B)The pH of blood is slightly basic. C)When the hydrogen ion concentration decreases, the hydroxyl ion concentration also decreases. D)When acids and bases are mixed, they react with each other to form water and a salt |
front 160 Which of the following statements is false? | back 160 A)Catalysts increase the rate of chemical reactions, sometimes while undergoing reversible changes in shape. B)Larger particles move faster than smaller ones and thus collide more frequently and more forcefully. C)Chemical reactions progress at a faster rate when the reacting particles are present in higher numbers. D)Chemical reactions proceed more quickly at higher temperatures. |
front 161 Select the most correct statement. | back 161 A)The endocrine system is not a true structural organ system. B)Organ systems operate independently of each other to maintain life. C)The immune system is closely associated with the lymphatic system. D)Organ systems can be composed of cells or tissues, but not both. |
front 162 Which of the following are survival needs of the body? | back 162 nutrients, water, atmospheric pressure, and oxygen |
front 163 One of the functional characteristics of life is excitability or responsiveness. This refers to | back 163 Sensing changes in the environment and then reacting or responding to them |
front 164 What does the "principle of complementarity of structures and function" mean? Answer: | back 164 What a structure can do depends on its specific form, or "structure determines function." |
front 165 ______ is explained by chemical and physical principles and is concerned with the function of specific organs or organic systems. Answer: | back 165 Physiology |
front 166 Which of the following imaging devices would best localize a tumor in a person's brain? | back 166 MRI |
front 167 _ _ physiology concerns urine production and kidney function. Answer: | back 167 Renal |
front 168 What is the function of the serous membranes? Answer: | back 168 They act to reduce friction and allow the organs to slide across cavity walls. |
front 169 Fully describe the anatomical position for the human body. Answer: | back 169 The body is erect, arms hanging at the sides, palms forward, and thumbs pointed away from the midline. |
front 170 Which of the following statements is the most correct regarding homeostatic imbalance? | back 170 It is considered the cause of most diseases. |
front 171
| back 171
|
front 172
| back 172
|
front 173 
| back 173 
|
front 174
| back 174
|
front 175
| back 175
|
front 176 
| back 176 
|
front 177 a type of bond important in tying different parts of the same molecule together into a three-dimensional structure | back 177 hydrogen bond |
front 178
| back 178
|
front 179 electrically charged particle from gain of an electron | back 179 anion |
front 180 
| back 180 
|
front 181 electrically charged particle from loss of an electron | back 181 CATION |
front 182
| back 182
|
front 183
| back 183
|
front 184
| back 184
|
front 185
| back 185
|
front 186
| back 186
|
front 187
Protein Synthesis | back 187
Protein Synthesis |
front 188
| back 188
Protein Synthesis |
front 189  1 ) Division of the cytoplasm. 2) Replication of chromosomes. 3) Chromosomes appear as threadlike bodies. 4) Chromatids move toward ends of spindle. | back 189 
|
front 190
| back 190
|
front 191
| back 191
|
front 192
| back 192
|
front 193 The source of energy captured in ATP. ______. | back 193 Glucose |
front 194 The building blocks of nucleic acids are | back 194 Nucleotides |
front 195 
| back 195 
|
front 196
| back 196
|
front 197 The _____ system plays a role in moving fluids, wastes, and bones? | back 197 Excretory |
front 198 The lower ribs are below the _____ region | back 198 Hypochondriac |
front 199 Sum of all CHEMICAL PROCESSES that keep our bodies alive and healthy. It is divided into 2 phases (parts): CATABOLISM & ANABOLISM: | back 199 METABOLISM |
front 200  The central abdominal area is the _____ region. | back 200  Hypogastric |
front 201  A _____ fracture occurred in the elbow area. | back 201  dorsum |
front 202  The process of turning molecules that are ingested into forms that are compatible with the organism is _____. | back 202  Assimilation |
front 203  The force that water exerts on a system is referred to as the ________. | back 203  Hydrostatic pressure |
front 204 a vertical line which divides the body into a left section and a right section. | back 204 Sagittal plane |
front 205 a vertical line which divides the body into a front (anterior) section and back (posterior) section. | back 205 Coronal plane |
front 206  a horizontal line which divides the body into an upper (superior) section and a bottom (inferior) section. The plane divides the body into top and bottom. | back 206  Transverse plane |
front 207 subunits that make up nucleic acids | back 207 Nucleotides |
front 208 the chemical bond that links two amino acids by connecting the amino group of one amino acid to the acid group of another | back 208 Peptide bond |
front 209 a lipid containing phosphate group | back 209 phospholipids |
front 210 a molecule made of many similar subunits | back 210 Polymer |
front 211 a long chain of amino acids | back 211 polypeptide |
front 212 one of two kinds of information-carrying nucleic acids found in cells; ribonucleic acid | back 212 RNA |
front 213
| back 213
|
front 214 compounds composed of a glycerol molecule with three fatty acids attached | back 214 Triglycerides |
front 215 adenosine triphosphate; a nucleotide composed of an adenine base, a sugar, and three phosphate groups | back 215 adenosine triphosphate (ATP) |
front 216 pairs of complementary nucleic acid bases; that is, A and T or C and G | back 216 base pair |
front 217 a polymer of nucleotides with the bases adenosine, guanine, cytosine, and thymine; deoxyribonucleic acid | back 217 DNA |
front 218
| back 218
|
front 219 a hydrocarbon chain with a carboxylic acid group at one end. | back 219 Fatty Acids |
front 220
| back 220
|
front 221
| back 221
|
front 222 fatty molecules that dissolve poorly in water but dissolve well in a nonpolar solvent; fats and oils | back 222 Lipids |
front 223 Apoptosis is programmed cell suicide; cancer cells do not undergo this process | back 223 TRUE |
front 224 key information-carrying molecules in cells | back 224 nucleic acids |
front 225 The coronal plane divides the body into _____ and _____ portions. | back 225 Posterior and anterior |
front 226
The plane divides the body into left and right
portions. | back 226 MEDIAN OR MIDSAGITAL |
front 227 TOWARDS THE POINT OF REFERENCE | back 227 PROXIMAL |
front 228 The plane divides the body into front and back. | back 228 FRONTAL OR CORONAL |
front 229
ORGANS FOUND IN THE ABDOMINAL
CAVITY | back 229 STOMACH, LIVER, SPLEEN, PANCREASE, PART OF THE LARGE INTENSITNE, PART OF SMALL INTESTINE. |
front 230
DISTAL | back 230 AWAY FROM THE POINT OF REFERENCE |
front 231
lateral | back 231 Towards the side |
front 232
ORGANS FOUND IN THE PELVIC CAVITY | back 232 UTERUS, BLADDER, OVERIES |
front 233
CORONAL | back 233 ANOTHER NAME FOR THE FRONTAL PLANE |
front 234
SUPERFICIAL | back 234 TOWARDS THE SURFACE |
front 235 What must happen before a cell can begin mitosis? | back 235
The chromosomes must be duplicated. |
front 236 The centrosomes move away from each other and the nuclear envelope breaks up during which phase of mitosis? | back 236 prophase |
front 237 The chromosomes line up in the center of the cell during which phase of mitosis? | back 237 Metaphase |
front 238 The sister chromatids separate and begin moving toward opposite poles of the cell during which phase of mitosis? | back 238 Anaphase |
front 239 The chromosomes arrive at the poles and nuclear envelopes form during which phase of mitosis? | back 239 Telophase |
front 240
The spindle now can move into the center of the cell.
| back 240 Prometaphase |
front 241 The chemical symbol O=O means | back 241 the atoms are double bonded |
front 242 What does CH4 mean? | back 242 There is one carbon and four hydrogen atoms. |
front 243
| back 243
|
front 244 An atom with a valence of 3 may have a total of ________ electrons. | back 244 NUMBER 13 |
front 245 AB → A + B is an example of a(n) ________ reaction. Answer: | back 245 Decomposition |
front 246 Homogeneous, will not settle. | back 246 SOLUTIONS |
front 247 Sucrose is a? | back 247 disaccharide |
front 248
| back 248
|
front 249
| back 249
|
front 250 Choose the anatomical topic and definition that is not correctly matched. | back 250 Cytology: study of the structures in a particular region. |
front 251 Which of the following is an example of a suspension? | back 251 BLOOD |
front 252 In which quadrant of the abdominopelvic cavity is the stomach located? | back 252 LEFT UPPER QUADRANT |
front 253 eicosanoids | back 253 REGULATE INFLAMMATION |
front 254
| back 254
|
front 255 What major chemical is responsible for apoptosis? | back 255 caspases |
front 256 Energy that travels in waves. Part of the electromagnetic spectrum. | back 256 RADIANT ENERGY |
front 257 Amino acids joining together to make a peptide is a good example of a(n) ______ reaction. | back 257 synthesis |
front 258 molecule and compound | back 258 mean the same thing and can be used interchangeably. |
front 259 A chemical bond is an energy relationship between outer electrons and neighboring atoms. | back 259 TRUE |
front 260 Microtubules are hollow tubes made of subunits of the protein tubulin. | back 260 TRUE |
front 261 In the compound H2CO3, what do the numbers 2 and 3 represent? | back 261 The 2 indicates that there are two hydrogen atoms in the compound and the 3 indicates that there are three oxygen atoms in the compound. |
front 262 The most common extracellular ion is ________. Answer: | back 262 sodium |
front 263 Which of the following is a function of a plasma membrane protein? | back 263 molecular transport through the membrane |
front 264 A process by which large particles may be taken into the protection of the body by invaders like bacteria, or for disposing of old or dead cells is called phagocytosis | back 264 TRUE |