Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Experiment 24: Theory Dibenzalacetone Synthesis (final review)
front 1 How is the synthesis of dibenzalacetone classified according to reaction type? | back 1 Aldol condensation. |
front 2 Write the balanced equation for the overall preparation of dibenzalacetone. | back 2  acetone + 2 benzaldehyde --OH--> dibennzalacetone + 2 H2O (CH3)2CO + 2 C6H5-CHO –OH-->C6H5HC=CH-CO-CH=CH-C6H5 + 2 H2O |
front 3 Why does the acetone carbanion react with benaldehyde rather than another molecule of acetone? | back 3 Benzaldehyde is more active than acetone. |
front 4 Why is this reaction carried out in a solvent mixture that contains both water (in which 10% NaOH is dissolved) and alcohol? Why can't either solvent be used alone. | back 4 Water is used to dissolve the NaOH and alcohol is used to dissolve benzaldehyde. If both benzaldehyde and NaOH aren't dissolved, they won't react. |
front 5 Two resonance structures for carbanion produced when NaOH reacts with acetone. Acetone carbanion. | back 5 |
front 6 What should be the approximate location of C=O in the IR spectrum of pure dibenzalacetone? | back 6 1649 cm-1 |
front 7 What happens if too much acetone is used the synthesis of dibenzalacetone? | back 7 Too much acetone would produce benzalacetone as opposed to dibenzalacetone. |
front 8 What hapens if the reaction flask isn't stoppered and some of the acetone evaporates during the synthesis of dibenzalacetone? | back 8 If acetone evaporates, there will not be enough acetone to contribute enough alpha hydrogens, preventing the reaction from occurring. |
front 9 What happens if the product crystals are filtered immediately upon formation? | back 9 Crystallization would be incomplete leading to a loss of product. |
front 10 acetone + NaOH ---> ? | back 10 + H2O |
front 11 benzaldehy + NaOH (conc.) | back 11  2 C6H5CHO + NaOH → C6H5CO2Na + C6H5CH2OH |
front 12 What are alpha carbons? | back 12 http://science.uvu.edu/ochem/index.php/alphabetical/a-b/alpha-carbon/ |
front 13 Benzaldehyde | back 13 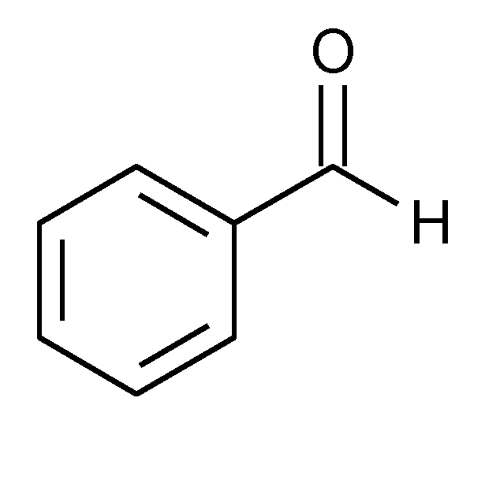 |
front 14 Dibenzalacetone | back 14 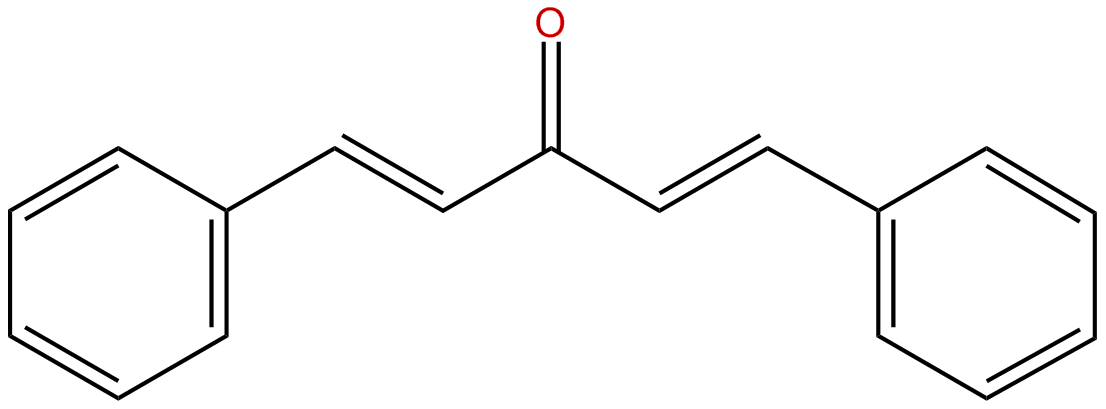 |
front 15 acetophenone + benzaldehyde + NaOH (2:1 ratio) | back 15  ????? |
front 16 Calculate theoretical yield. | back 16  |
front 17 Calculate limiting reactant. | back 17  1. calculate theoretical yield 2. lowest yield is limiting reactant |
front 18 Calculate percent yield. | back 18 (actual / theoretical) x 100 |
front 19 What is aldol condensation? | back 19 Enolizable aldehydes and enolizable ketones, in the presence of an acid or base catalyst in aqueous medium at high temperature, undergo a reaction, giving an α, β-unsaturated aldehyde or an α, β-unsaturated ketone, respectively, as the product. |
front 20  Resources | back 20 http://faculty.mdc.edu/qzhang/chm2211L/experiment24.htm https://www.youtube.com/watch?v=YQkcNeIgdoU http://course1.winona.edu/tnalli/s13/expt6dibenzalacetone.html |