Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 1: Measurement and Energy
front 1 Classify each of the following statements as a hypothesis, theory, or law. | back 1 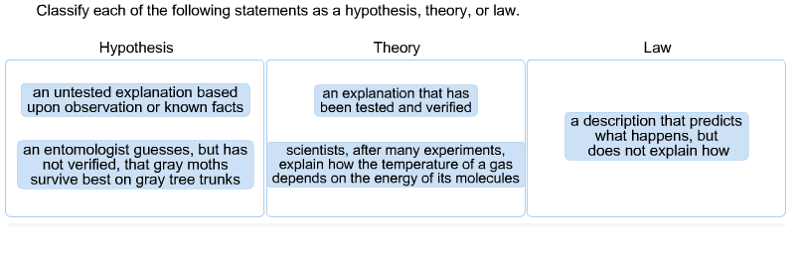 A hypothesis is an educated guess, or an untested explanation, that is based upon observations or know facts. For example, if an entomologist guesses that gray moths survive better on gray tree trunks, he or she has constructed a hypothesis. This hypothesis can be tested in one or more experiments or tests.
|
front 2 Which of the following are chemical changes? Check all that apply. | back 2  The only chemical change in the list is <"gasoline is burned>".
|
front 3 Give the SI base unit of each of these quantities. Enter the abbreviation rather than the name of the unit. | back 3  The SI base unit of mass is the kilogram, kg.
|
front 4 Identify the power of ten that defines each of these prefixes. | back 4  The powers of ten that define each of these prefixes are:
|
front 5 Write the following numbers in scientific notation. | back 5  673.5 = 6.735 × 10^2
|
front 6 Rank these values according to magnitude. | back 6 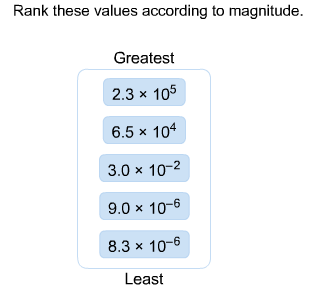 First, rank the values by exponent:
|
front 7 Convert the following length to its equivalent in nanometers. | back 7 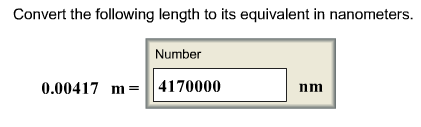 A nanometer is a billionth of a meter:
|
front 8 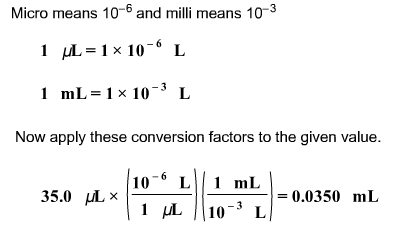 Convert the following volume to its equivalent in milliliters: | back 8  |
front 9  Convert 3.93 μm to inches. | back 9 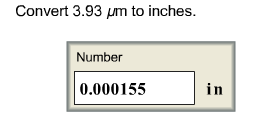 |
front 10 Each of the measurements below describes length. Rank them from longest length to shortest length. | back 10  It is easier to rank these by first converting them to a single unit. An example conversion (to meters) is worked below:
|
front 11  Liquid helium boils at –268.93 °C. What is the boiling point of helium on the Kelvin temperature scale? | back 11  |
front 12  Many bacteria, such as E. coli, thrive at body temperature, 37.0 °C; therefore, bacteria are grown in laboratories at that temperature. What is the corresponding Fahrenheit temperature? | back 12  |
front 13 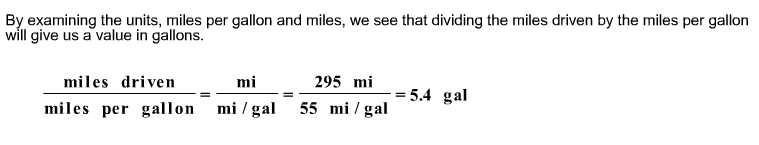 A certain hybrid car has a mileage rating of 55 miles per gallon. If the car makes a trip of 295 miles, how many gallons of gasoline will be used? | back 13  |
front 14 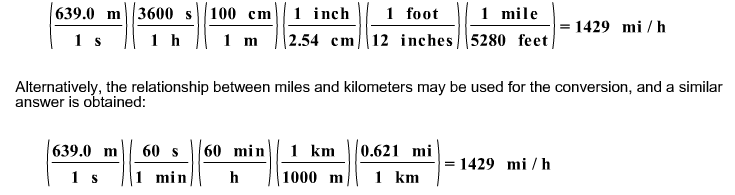 If a bullet travels at 639.0 m/s, what is its speed in miles per hour? | back 14  |
front 15 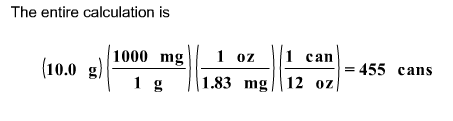 If the caffeine concentration in a particular brand of soda is 1.83 mg/oz, drinking how many cans of soda would be lethal? Assume 10.0 grams of caffeine is a lethal dose, and there are 12 oz in a can. | back 15  First, we calculate the number of mg of caffeine in a can of soda.
|