Purpose of Experiment 7?
to illustrate typical techniques used in gravimetric analysis
by quantitatively determining
the amount of chloride in an unknown
What is quantitative analysis?
aspect of analytical chemistry concerned with determining
how much of one or more constituents is
present in a sample of material
Two common quantitative methods used in analytical chemistry
Gravimetric analysis
Volumetric analysis
Gravimetric analysis

derives name from the fact that
the constituent being determined can be in isolated
in some weighable form
Volumetric Analysis
.jpg)
derives name from the fact that
the method used to determine amount of constituent involves
measuring the volume of a reagent
What are the steps involved in gravimetric analysis (7)?
(These are not steps, they are not are in order)
1. Dry and weigh samples of material
2. Dissolve the samples
3. Precipitate the constituent (substance you are trying to attain) by adding a suitable reagent
4. Isolate the precipitate by filtration
5. Wash precipitate to free it of contaminants
6. Dry the precipitate (to obtain weighable form)
7. Calculate the percentage of the desired consituent (from masses of the sample and precipitate)
Example of precipitation reaction

a chloride ion may be precipitated by adding the silver ion to make AgCl
- AgCl is very insoluble, so adding Ag to Cl precipitates AgCl quantitatively
(the precipitate can be collected, dried, and weighed)
From the amount of AgCl obtained, amount of Cl can be calculated
Stoichiometry
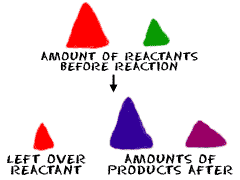
the determination of the proportions in which elements combine
and the mass relations in any chemical reaction
gravimetric factor

- converts gram of a compound into grams of a single element
- used repeatedly in analytical chemistry and are tabulated in books
Example of Gravimetric Analysis

Which acid was used in this procedure?
Which silver compound was used?
HNO3 - nitric acid
AgNO3- silver nitrate (.25M instead of .5 M)
How was the filter paper placed into the funnel?
open the paper so that it has one piece of paper against one side of the funnel and three pieces of paper against the other
- wet the paper w/ distilled water to hold it in place
Which compound was highly flammable and needed to be kept away from the flames?
acetone
What were the instructions for storing the AgCl precipitate? Why?

Keep it out of bright light
because it is photosensitive and slowly decomposes in the presence of light
(hv= symbol for electromagnetic radiation
What is the purpose of standard deviation?
to estimate the precision of your results
The smaller the deviation, the more precise the measurements
accuracy
correctness of measurement,
closeness to true result
precision
internal consistency among one's own results (reproducibility)
error
difference btwn the true result and the determined result
Determinate errors
errors in method or performance that can be discovered and eliminated
*these errors are known and are controllable (e.g.instrumental errors, human errors)
Indeterminate errors
random errors, which are incapable of discovery but which can be treated by statistics
*these errors are unknown and beyond analysts' control (e.g. room temperature
mean
arithmetic mean or overage (μ)
median

midpoint of results (if odd number of results)
average of two middle results (if even number of results)
How is relative deviation calculated?
by dividing the average deviation from mean by the mean
What is the best measure of precision
standard deviation
What is the rule for retaining or discarding a standard deviation figure?
discard any result that is more than two standard deviations away from the mean