What are the five types of Chromatographic separation?
-Adsorption chromatography
-Partition chromatography
-Ion Exchange chromatography
-Size exclusion chromatography
- Affinity Chromatography
Solute equilibrates between mobile phase and surface of stationary phase
Adsorption Chromatography
Solute equilibrates between mobile phase and film of liquid attached to stationary phase
Partition chromatography
Ions in mobile phase are attracted to counterions covalently attached to stationary phase
Ion exchange chromatography
Different-sized solutes penetrate pores in the stationary phase to different extents. The largest solutes are eluded first
Molecular (size) exclusion chromatography
Molecular exclusion chromatography separates by size, so the large molecules______, and the solid stationary phase has a_____gel
elude first, porous
What is the mobile phase?
-the solvent moving through the column
- a liquid or gas
What is the stationary phase?
-the one that stays in place inside the column
- a viscous liquid chemically bonded to the inside of a capillary tube or packed column
In Ion exchange chromatography, what eludes first in an anion exchange chromatography?
The positively charged molecules will elude first because the stationary phase attracts the negatively charged molecules.
In Ion exchange chromatography, what eludes first in an cation exchange chromatography?
The negatively charged molecules will elude first because the stationary phase attracts positively charged molecules.
Retention time, tr is the
elapsed time between injection and arrival
Retention volume, Vr, is the volume of
mobile phase required to elute a particular solute from the column
Gaussian chromatography states that if the peaks are farther apart,
the better the separation
Gaussian chromatography states that the longer the peak resides in a column,
the broader the band width
A resolution____ than 1.5 is highly desireable
greater
The narrower the peaks are, the____the separation, and the ___the resolution
better, higher
How does the column length affect the resolution?
increasing the column length allows for more separation time, and increases retention time and run time
How does temperature affect resolution?
raising the temperature in GC decreases the retention time by reducing the partition coefficient, allowing for faster elution. Higher temps can also reduce resolution and cause peaks to overlap
how does coating thickness affect resolution?
A thicker stationary phase will increase the retention time by providing more interaction time for analytes, enhancing the separation. Too thick can lead to excessive broadening and reduced efficiency
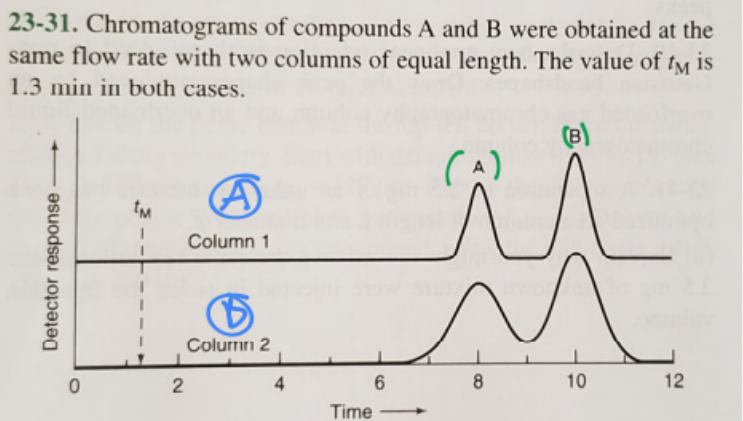
Which column has a larger plate number?
A(blue)
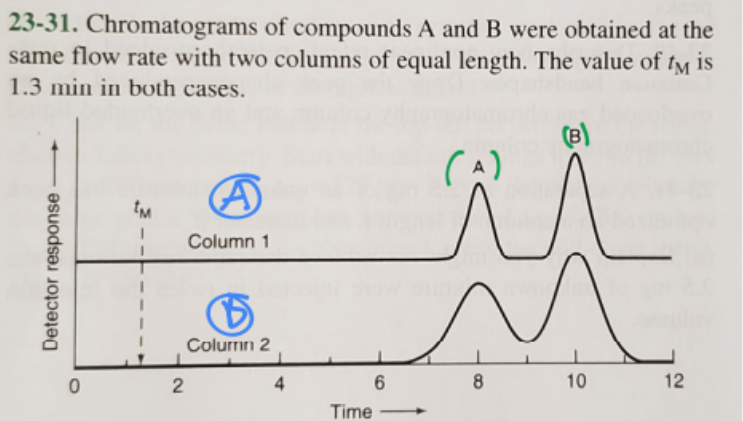
Which column has a larger plate height?
B (blue)
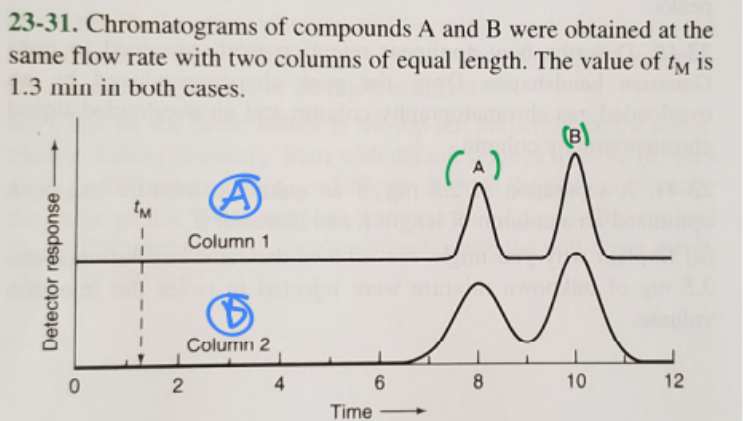
1. Which column gives higher resolution?
2. Which column gives a greater separation factor?
3. Which compound has a higher retention factor?
1. A (blue)
2. A (blue)
3. B (green)
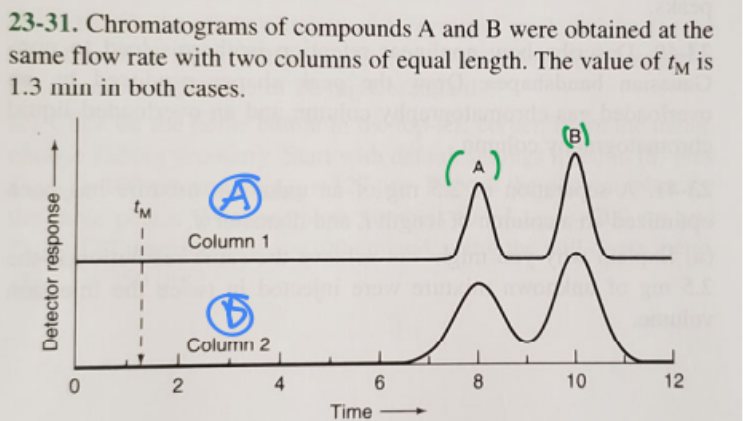
1. Which compound has a greater distribution constant?
B (green)
In Normal Phase HPLC, the polar column allows for polar analytes to have
longer retention times, so non-polar molecules eludes first
In Reverse Phase HPLC, the non-polar column allows for non-polar analytes to have
longer retention times, so polar molecules elude first
What does each letter stand for? H=A+(B/ux)+Cux
____ plate height
____Eddy diffusion (multiple path)
____longitudinal diffusion
____ linear flow rate of mobile phase
_____resistance mass transfer in stationary and mobile phases
H , A, B, ux, C
A study of interaction between matter and radiation
Spectroscopy
Technique that uses light to measure chemical concentrations
spectrophotometry
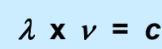
What does each symbol stand for?
lambda: wavelength (nm)
V: frequency (sm-1)
c: speed of light (3.0x108m/s)
Electronic transition occurs when
molecules absorb UV or visible light, causing electrons to move to higher electronic energy levels
Vibrational transition occurs in the
infrared region, causing them to vibrate
Rotational transition occurs in the microwave region causing
molecules to rotate

Label the following picture of measuring absorbance:
1. light sources
2. wavelength selector (monochromator)
3. sample
4. Light detector
Absorption spectrophotometer measures
how much light is absorbed by a sample at specific wavelengths
Emission spectrometer measures
how much light emitted by a sample after it has been excited
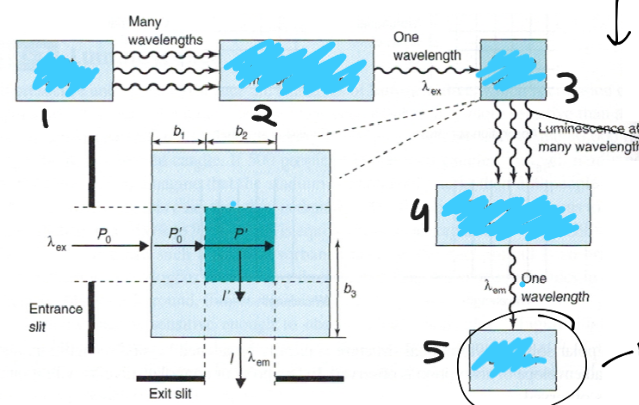
Label the following for measuring emission:
1. light source
2. excitation monochromator
3. sample cell
4. emission monochromator
5. detector
Beers law: A=ε⋅b⋅C, what do each letters signify?
A= absorbance
b= path length of the sample cuvette
c=concentration of solution
You can find concentration by relating these 2 equations: A=ε⋅b⋅C and Y=mx+b. What symbols work hand in hand?
y= A
m=ε
x=b
b=C
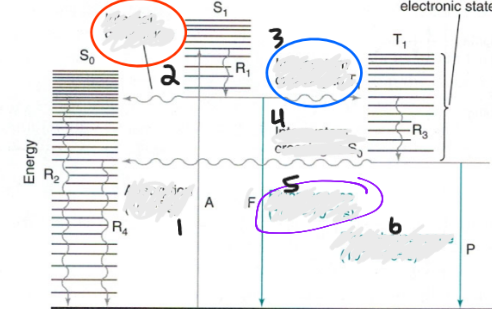
Label Jablonski Diagram:
1. absorption (1015s)
2. Internal conversion
3. intersystem crossing to T
4. INtersystem crossing to So
5. Fluorescence (10-8-10-4 s)
6. Phosphorescence (10-4-102 s)
Has shorter emission time, brighter emission, emission of photon during internal converion, and only glows when UV light hits it
Fluorescence
longer emission time, dimmer emission, emission of photon during intersystem crossing, and can be charged with UV light
Phosphorescence
Internal conversion is a nonradioactive transition between states with the
same spin
Intersystem crossing is a nonradioactive transition between states with
different spins
The process by which an atom or molecule loses electrons
Na → Na+ + e−
Oxidation
The process by which an atom or molecule gains electrons.
Cl2 + 2e− → 2Cl−
Reduction
This is the substance that gains electrons ( gets reduced)
while it causes another substance to be oxidized.
oxidizing agent
This is the substance that loses electrons ( gets oxidized)
while it causes another substance to be reduced.
reducing agent
Galvanic cells are spontaneous and
external energy is not required
In a reaction equation, the left is the____ and the right is the____
anode, cathode
The anode gets______ and the cathode gets ____
oxidized, reduced
If E>0, it is a _____reaction, and if E<0, it is a _____reaction
spontaneous, non-spontaneous