Where should the tip of the thermometer be placed in a microscale distillation set up?
At or slightly below the side arm of the distillation head.
What are the concerns presented by overheating a distillation to a dry flask?
- the empty glassware might heat quickly, igniting vapors from the distillation
- the remaining solid residue might contain explosive peroxides
When performing the acid-catalyzed dehydration of an alcohol, the yield of product can be increased by ___________________ during the reaction.
distilling off the product
The dehydration is ______________, and removing the product from _________________ reduces the ___________ reaction.
reversible, the reaction flask, hydration
Why does the dehydration of an alcohol more often use concentrated sulfuric acid, H2SO4, as the acid catalyst rather than dilute hydrochloric acid, HCl?
- The presence of the chloride ion could result in a competing substitution
- The additional water solvent from a dilute solution could reverse the dehydration.
Which changes can be made to a chemical reaction in order to increase the rate constant?
Add a catalyst, increase the temperature
Suppose you are performing a titration, at the beginning of the titration you read the titrant volume as 2.03 mL. After running the titration and reaching the endpoint, you read the titrant volume as 20.03 mL. What volume, in ML, of titrant was required for the titration
18.0 mL
For a reaction rate based on one reactant, identify the type of graph that will give a linear plot in each case.
first-order reaction __________________
zero order reaction __________________
second order reaction ______________
In[reactant] versus time
[reactant] versus time
1/[recatant] versus time
Concnetration data is commonly monitored duringa reaction to determine the order with respect to a reactant. Consider the types of observations listed, and determine which order is likely for that reactant. Assume all other factors are held constant.
The reaction rate is constant regardless of the amount of reactant in solution. ______________________
The reaction rate increases in direct proportion to the concentration of the reactant in solution. _________________
An increase in the concentration of the reactant in solution causes the reaction rate to increase exponentially.
zero order, first order, second order
The term SN1 describes a reaction that is a(n) __________________ involving _______________________________________
nucleophilic substitution, one molecule in the rate determining step
If the dehydration reaction of an alcohol is successful, what changes would be seen in the IR spectrum fro the product compared to the starting material?
- The disappearance of an O-H band from the starting material
- The disappearance of a C-O band from the starting material
-The addition of a C-C double bond band in the product
In the presence of acid and heat, ______________________ will undergo a more effective dehydration reaction because it is a _____________ alcohol and the ________ in the intermediate of the mechanism will be more stable.
cyclohexanol, secondary, cation
Describe the complete role of the acid catalyst in the dehydration of an alcohol.
The acid protonates the hydroxyl group and then the conjugate base deprotonates an adjacent carbon
How can a catalyst be recognized in a mechanism?
The catalyst is used and then regenerated in a later step
The dehydration of a secondary alcohol, like cyclohexanol, is a mechanism that occurs ____________________. First, the alcohol is protonated to _______________________________, creating ___________ intermediate. Then, a _________________ is removed, moving the electrons from that bond to make a __________________
in two steps, leave as a water molecule, a cation, hydrogen ion, carbon-carbon double
Why is potassium hydroxide preferred over sodium hydroxide in organic reactions?
Potassium hydroxide is more soluble in organic alcohols
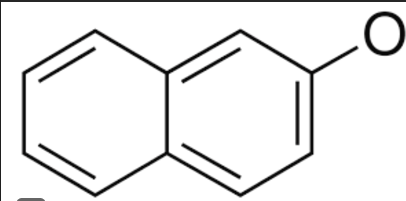
Identify the best description of the role each species play in the SN2 synthesis of nerolin
I- ______________________
KOH ____________________
I-CH2CH3 ___________________
(two rings w/ one O-) _____________
leaving group, base, electrophile, nucleophile
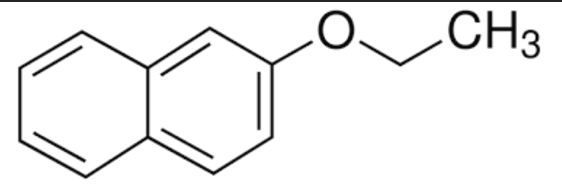
Identify the best description of the role each species play in the SN2 synthesis of nerolin
_____________________
product
Consider the SN2 reaction of an alcohol with an alkyl halide in the presence of base.
The ______ should be added to the alcohol first to _____________ the alcohol and allow it to attack the ______________
base, deprotonate, alkyl halide
An SN2 reaction is a type of ______________________ in which the nucleophile attacks the electrophile ____________________ a leaving group leaves.
The rate of the reaction depends on the concentration of _______________
substitution reaction, at the same time, both reactants
When working in a fume hood, what is the best position of the hood sash?
As low as possible no more than halfway up
When working in the fume hood, it is important to make an effort to minimize __________. Only keep items in the fume hood if they are being used for ________________. Do not _______ chemicals in the fume hood unless instructed to do so.
clutter, the current experiment, store
If crystal growth does not start on its own after the solution in the flask returns to room temperature, identify the best way to promote its process.
Add a bit of solid as a seed crystal, scratch the bottom of the flask gently with a stirring rod.
When should you start timing the reflux for the procedure?
when the reflux ring stabilizes in the condenser
When it is time to end a reflux, first _______________________ and then turn off the heat. ____________________________ until the system has cooled.
lower the heat source, leave the condenser water on
The biggest concern of working with hydrocarbons in lab is that many of them are _____________
Therefore, hydrocarbons should be handled _____________ to contain any associated vapors with that hazard
flammable, in the hood
Bayer's test, also known as the permanganate test, shows the presence of _______________
a positive bayers test appears as _____________
a negative bayers test appears as ______________
alkenes, a brown precipitate, a purple solution
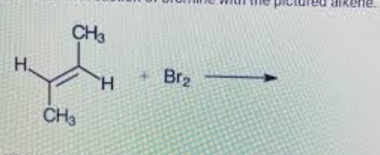
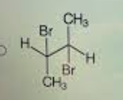
Consider the reaction of bromine with the pictured alkene
Which structure shows the correct bonding for the product?
The bromine test shows the presence of ____________
A positive bromine test appears as a _____________
A negative bromine test as ____________________
alkenes, a colorless solution, an orange solution
In general, when hydrocarbons like oil are added to water, the two liquids ______________ because hydrocarbons are ____________ and water is ___________
do not mix, non polar, polar
In general, when a hydrocarbon is added to water, the hydrocarbon will _________ the water because hydrocarbons are _______ than water
float above, less dense
A hydrocarbon is saturated if _________________________ are present.
_______________ are saturated hydrocarbons
A hydrocarbon is unsaturated if _____________________ are present.
_________________ are unsaturated hydrocarbons
only single bonds
Alkanes
any multiple bonds
Alkenes
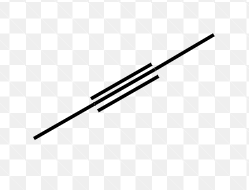
Identify the type of hydrocarbon represented by each structure
Alkyne (unsaturated)
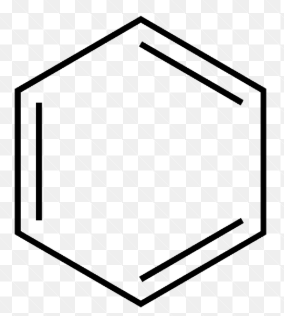
Identify the type of hydrocarbon represented by each structure
Aromatic (unsaturated)

Identify the type of hydrocarbon represented by each structure
Alkane (saturated)