1
isomer
each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
2
enantiomer

mirror image molecules, same molecular formula
3
structural isomer
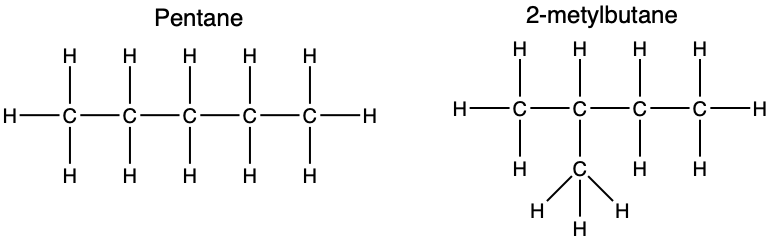
one of two or more compounds that contain the same number and kinds of atoms but that differ significantly in their geometric arrangement
4
cis-trans isomer
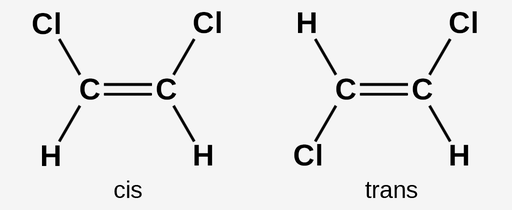
geometric isomers, where the functional group is placed differently with regards to the double bond.
cis isomer has molecules on one side of the double bond. A trans isomer has molecules on the other side of the double bond